Org. Synth. 2000, 77, 198
DOI: 10.15227/orgsyn.077.0198
α-TOSYLBENZYL ISOCYANIDE
[
Benzene, 1-[(isocyanophenylmethyl)sulfonyl]-4-methyl-
]
Submitted by Joseph Sisko
1
, Mark Mellinger
1
, Peter W. Sheldrake
2
, and Neil H. Baine
1
.
Checked by Yan Dong and Steven Wolff.
1. Procedure
A.
p-Toluenesulfinic acid
(1). A 2-L Erlenmeyer flask equipped with a magnetic stir bar is charged with 670 mL of water and
134.1 g (536 mmol) of p-toluenesulfinic acid, sodium salt
(Note 1) and the mixture is stirred for 20-30 min until a clear solution is obtained.
tert-Butyl methyl ether (TBME, 670 mL) is added followed by the slow addition of
44 mL of concentrated aqueous hydrochloric acid (HCl) (536 mmol) over 5 min. The mixture is stirred for an additional 20-30 min, transferred to a separatory funnel and the aqueous layer is removed. The organic layer is diluted with
670 mL of toluene
and concentrated with a rotary evaporator
(Note 2) until approximately 70-90% of the solvent has been removed.
Heptane (200 mL) is added and the white solid is collected by filtration using a Büchner funnel, rinsed with
270 mL of heptane
and dried under vacuum for 2-4 hr (Note 2) to give 71-76 g (85-91%) of
p-toluenesulfinic acid
(Note 3) that is used in the next step (Note 4).
B.
N-(α-Tosylbenzyl)formamide
(2). A 1-L, three-necked, round-bottomed flask fitted with an overhead stirrer, a reflux condenser capped with a nitrogen (N2) inlet and a temperature probe is charged with
55 mL of acetonitrile
and
55 mL of toluene
(Note 5),
10.7 mL (105.6 mmol) of benzaldehyde
(Note 6),
10.5 mL (264 mmol) of formamide
(Note 6) and
14.7 mL (116 mmol) of chlorotrimethylsilane
(Note 6). After heating the solution at 50°C for 4-5 hr,
24.7 g (158.3 mmol) of p-toluenesulfinic acid
(1)
(Note 7) is added and heating is continued for an additional 4-5 hr. The solution is cooled to room temperature and
55 mL of TBME is added. The solution is stirred for 5 min and 275 mL of water is added. The resulting mixture is cooled to 0°C, held there for 1 hr and the precipitated white solid is collected using a Büchner funnel. The reaction flask is washed with
35 mL of TBME and this rinse is poured over the filter cake. After washing with TBME a second time, the solid is dried in a vacuum oven at 60°C for 5-10 hr to give 26.6-29.1 g (85-94%) of
N-(α-tosylbenzyl)formamide
(2) which is used in the next step without further purification (Note 8).
C.
α-Tosylbenzyl isocyanide
(3)
(Note 9). A 1-L, three-necked, round-bottomed flask fitted with an overhead stirrer, a 100-mL addition funnel and a temperature probe is charged with
200 mL of tetrahydrofuran (THF)
(Note 10) and
27.6 g (94.8 mmol) of N-(α-tosylbenzyl)formamide
(2).
Phosphorus oxychloride (17.7 mL, 190 mmol)
(Note 11), (Note 12) is added and the resulting solution is stirred for 5 min at 25°C. After cooling the solution to 0°C,
79.3 mL (569 mmol) of triethylamine
(Note 11), (Note 12) is added slowly over 30-45 min while keeping the internal reaction temperature below 10°C. After the triethylamine addition is complete, the reaction is warmed to 5-10°C and held there for 30-45 min.
Ethyl acetate (140 mL) and water (140 mL) are added sequentially to the reaction, the mixture is stirred for 5 min and, after transferring the mixture to a separatory funnel, the aqueous layer is removed. The organic layer is washed with water (2 × 140 mL), saturated
sodium bicarbonate (NaHCO3) solution (140 mL) and
brine (70 mL). The organic layer is transferred to a 500-mL, round-bottomed flask and concentrated on a rotary evaporator (Note 13). The residue is diluted with
140 mL of 1-propanol
(Note 11), (Note 14) and this solution is concentrated on a rotary evaporator to half of its original volume. The residue is cooled to 5-10°C for 30 min and the beige solid that crystallizes is filtered through a Büchner funnel. The filter cake is rinsed twice with
75 mL of 1-propanol
. The beige solid is dried under vacuum for 3-4 hr (Note 13) to give 18.1-19.7 g (70-76%) of
α-tosylbenzyl isocyanide
(3)
(Note 15).
2. Notes
1.
p-Toluenesulfinic acid, sodium salt, purchased from the Aldrich Chemical Company, Inc.
, was used without purification and was found to be a tetrahydrate.
2.
Minor decomposition of
p-toluenesulfinic acid is observed at temperatures above 55°C. For this reason, the water bath of the rotary evaporator and all subsequent heating operations should be kept at temperatures below 35-40°C to obtain best results.
3.
p-Toluenesulfinic acid should be used immediately after its preparation or stored under N
2 and used within 2-3 weeks.
4.
The spectra are as follows:
1H NMR (300 MHz, DMSO-d
6) δ: 2.36 (s, 3 H), 7.35 (d, 2 H, J = 8.1), 7.54 (d, 2 H, J = 8.1)
;
13C NMR (75 MHz, DMSO-d
6) δ: 18.7, 122.9, 127.9, 140.85, 143.6
.
5.
Reagent grade
toluene
and
acetonitrile supplied by J.T. Baker Inc.
were used without further purification.
6.
Fresh bottles of
benzaldehyde (99%), formamide (99+%) and chlorotrimethylsilane (98%) from the Aldrich Chemical Company, Inc.
, were used without further purification.
7.
The
50 mol % excess of p-toluenesulfinic acid
is required to obtain the best results. Using only
10-20 mole % excess of the sulfinic acid typically lowers the yield by 20-30%. This has been attributed to the known propensity of arylsulfinic acids to disproportionate.
3 The checkers noted the presence of insoluble material after the addition of
p-toluenesulfinic acid. This did not effect the yield.
8.
Compound
2 exists as a 5:1 mixture of amide rotamers at 25°C in DMSO-d
6. The spectra for the major rotamer are as follows:
1H NMR (300 MHz) δ: 2.40 (s, 3 H ), 6.38 (d, 1 H, J = 10.6), 7.42 (m, 5 H), 7.55 (m, 2 H), 7.70 (d, 2 H, J = 8.1), 7.96 (s, 1 H), 9.75 (d, 1 H, J = 10.6)
;
13C NMR (75 MHz, DMSO-d
6) δ: 21.0, 70.2, 128.1, 129.0, 129.1, 129.3, 129.45, 130.3, 133.4, 144.7, 160.1
; mp
172-173°C (lit.
4
160-162°C); IR (KBr) cm
−1: 3336, 1654, 1320, 1145
. Anal. Calcd for C
15H
15NO
3S: C, 62.27; H, 5.23; N, 4.84. Found C, 61.97; H, 5.11; N, 4.71.
9.
Step C is a modified literature procedure.
4
10.
A new bottle of
reagent grade THF (water content < 0.02%) from J. T. Baker Inc.
was used without further purification.
11.
Phosphorus oxychloride (99%), triethylamine (99%), and 1-propanol (99+%) were purchased from the Aldrich Chemical Company, Inc.
, and used without further purification. The checkers noted the presence of insoluble material after addition of the
phosphorus oxychloride.
12.
The excess amounts of
phosphorus oxychloride and
triethylamine are required to ensure that the reaction goes to completion. Using lesser amounts of these reagents leads to incomplete reactions.
13.
Isocyanides similar to
3 have been found to be thermally unstable at temperatures above 80°C. To secure a margin of safety, avoid heating
3 and similar isocyanides at temperatures above 35-40°C.
14.
The checkers noted formation of a suspension upon addition of the
1-propanol.
15.
The spectra are as follows: mp
145°C dec (lit.
4 128-130°C dec); IR (KBr) cm
−1: 2131, 1331, 1158
;
1H NMR (300 MHz, CDCl
3) δ: 2.45 (s, 3 H), 5.61 (s, 1 H), 7.39 (m, 7 H), 7.59 (d, 2 H, J = 8.2)
;
13C NMR (75 MHz, CDCl
3) δ: 21.75, 76.6, 126.7, 128.4, 128.7, 129.8, 130.3, 130.5, 130.7, 146.6, 166.4
. Anal. Calcd for C
15H
13NO
2S: C, 66.40; H, 4.83; N, 5.16. Found C, 66.42; H, 4.88; N, 5.13.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Tosylmethyl isocyanide (TosMIC) and its congeners are useful and versatile building blocks for the construction of heterocyclic molecules.
5 While TosMIC is available from a variety of commercial sources, substituted TosMIC reagents have suffered from the lack of general and efficient methods for their preparation.
6
The present procedure provides ready access to a variety of substituted TosMIC reagents, exemplified by
α-tosylbenzyl isocyanide
(3), by dehydration of the corresponding formamides
(2). While this route has been employed previously, the reported methods of preparation for formamides
2 suffer from low yields
7 and extended reaction times.
4 The present process for preparing these formamides offers high yields, mild conditions and generality, as shown below in the Table.
TABLE
PREPARATION OF SUBSTITUTED TOSMIC REAGENTS
|
|
|
|
Entry
|
R
|
% Yield
|
% Yield
|
|
|
1
|
4-F-C6H4
|
93
|
70-80
|
|
2
|
4-MeO-C6H4
|
92
|
74
|
|
3
|
3-Thiophene
|
81
|
76
|
|
4
|
Me2CHCH2
|
81
|
58
|
|
5
|
Me2CH
|
62
|
60
|
|
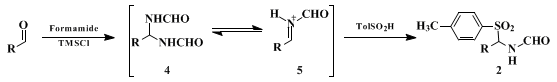
The reaction proceeds by formation of bisformamide 4, which can be isolated but is more typically formed and reacted in situ. The bisformamide 4 is presumably in equilibrium with the corresponding iminium ion 5, which is captured by the sulfinic acid to generate 2. The TMSCl consumes the water of condensation while liberating HCl, which catalyzes the entire sequence.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
α-Tosylbenzyl isocyanide:
Benzene, 1-[(isocyanophenylmethyl)sulfonyl]-4-methyl- (9); (36635-66-2)
p-Toluenesulfinic acid (8);
Benzenesulfinic acid, 4-methyl- (9); (536-57-2)
p-Toluenesulfinic acid, sodium salt (8);
Benzenesulfinic acid, 4-methyl-,
sodium salt (9); (824-79-3)
tert-Butyl methyl ether:
Ether, tert-butyl methyl (8);
Propane, 2-methoxy-2-methyl- (9); (1634-04-4)
N-(α-Tosylbenzyl)formamide:
Formamide, N-[[(4-methylphenyl)sulfonyl]phenylmethyl]- (9); (37643-54-2)
Benzaldehyde (8,9); (100-52-7)
Formamide (8,9); (75-12-7)
Chlorotrimethylsilane:
Silane, chlorotrimethyl- (8,9); (75-77-4)
Phosphorus oxychloride: HIGHLY TOXIC:
Phosphoryl chloride (8,9); (10025-87-3)
Triethylamine (8);
Ethanamine, N,N-diethyl- (9); (121-44-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved