Org. Synth. 2002, 78, 23
DOI: 10.15227/orgsyn.078.0023
PALLADIUM-CATALYZED AMINATION OF ARYL HALIDES AND ARYL
TRIFLATES: N-HEXYL-2-METHYL-4-METHOXYANILINE AND N-METHYL-N-(4-CHLOROPHENYL)ANILINE
[
Benzenamine, 4-chloro-N-methyl-N-phenyl-
]
Submitted by John P. Wolfe and Stephen L. Buchwald
1
.
Checked by Holly Norling and Louis S. Hegedus.
1. Procedure
A.
N-Hexyl-2-methyl-4-methoxyaniline
.
A 250-mL, round-bottomed flask equipped with a magnetic
stirbar and a rubber septum is flame-dried and
allowed to cool to room temperature under an argon purge. The
septum is removed and the flask is charged with
tris(dibenzylideneacetone)dipalladium(0)
(114 mg, 0.125 mmol, 0.5 mol% Pd)
(Note 1), (±)-BINAP
(233 mg, 0.375 mmol, 0.75 mol%)
(Note 1),
and
sodium
tert-butoxide (NaOtBu) (6.73 g, 70.0 mmol,
1.4 equiv)
(Note 2). The septum is again placed on
the flask, and the flask is purged with argon for 5 min.
Toluene (50 mL)
(Note 3) is added and stirring is started. The flask is charged with
4-bromo-3-methylanisole (10.0 g,
50.0 mmol, 1.0 equiv)
(Note 4),
n-hexylamine (7.9 mL, 60.0
mmol, 1.2 equiv)
(Note 5), and additional
toluene (50 mL).
The resulting dark red mixture is placed in an oil bath that
is heated to 80°C with stirring until the aryl bromide has been completely consumed
as judged by GC analysis (18-23 hr) (Note 6). The mixture is
removed from the oil bath, allowed to cool to room temperature,
then poured into a separatory funnel. The reaction flask is
rinsed with ether (2 × 50 mL), brine (100 mL), deionized water (20 mL), and
again with ether (50 mL). All rinses are added to the separatory funnel,
the funnel is shaken, and the layers are separated. The aqueous layer is extracted
with ether (50 mL), and the combined organic extracts are dried over anhydrous
magnesium sulfate
. The mixture is filtered;
the magnesium sulfate is washed with ether (50 mL), filtered,
and the organic solution is concentrated under reduced pressure to give the crude
product as a brown oil. This oil is then distilled (bulb-to-bulb, bp 92°C at 0.001 mm) to afford 10.35 g (94%)
of the desired product as a pale yellow oil (Note 7). A small
amount of viscous material remains in the distillation flask following the distillation.
B.
N-Methyl-N-(4-chlorophenyl)aniline
.
A 250-mL, three-necked, round-bottomed flask equipped with
a reflux condenser, magnetic stirbar,
one glass stopper, and rubber septa, covering the
condenser and the remaining neck of the flask, is flame-dried and allowed
to cool to room temperature under an argon purge. The septum
is removed and the flask is charged with
palladium
acetate (337 mg, 1.5 mmol, 3.0 mol% Pd)
(Note 1) and (±)-BINAP (1.4
g, 2.25 mmol, 4.5 mol%). The septum is again placed
on the flask, and the flask is purged with argon for 5 min. Tetrahydrofuran (THF) (50 mL)
(Note 8) is added and the mixture stirred at room temperature for 10 min
until a peach-colored suspension forms. The flask is charged with
4-chlorophenyl
trifluoromethanesulfonate
(13.0 g, 50.0 mmol, 1.0 equiv)
(Note 9), and N-methylaniline (6.5
mL, 60.0 mmol, 1.2 equiv)
(Note 5).
The septum is removed from the flask, and cesium carbonate
(Cs2CO3) (22.8 g, 70.0 mmol, 1.4
equiv)
(Note 10) is added under a flow of argon.
The septum is again placed over the flask, and the flask is purged with argon
for 30 seconds. Additional THF (50 mL) is added, the reaction mixture is immersed
in a 70°C oil bath so that the level of the oil is even with the level of solvent
in the flask and stirring is begun. The internal temperature of the reaction is monitored
using a thermocouple and is found to be 60°C (±1°C) (Note 11).
The mixture is stirred at this temperature until all the starting triflate has been
consumed as judged by GC analysis (23-45 hr) (Notes 6,
12). The mixture is removed from the oil
bath and allowed to cool to room temperature. Ether (100 mL) and hexanes
(50 mL) are added to the flask and the solution formed is filtered through Celite.
The flask is rinsed with ether (3 × 50 mL) and the rinses are filtered through
Celite. The organic extracts are combined and concentrated under reduced pressure,
and 1/1 (v/v) hexanes/ether (200 mL) is added. A yellow precipitate forms, and the
mixture is filtered through a 1.5-inch deep plug of silica gel on a 3"-diameter
type D fritted funnel. The flask is rinsed with 1/1 (v/v) hexanes/ether
(2 × 100 mL), and the rinses are filtered through the plug of silica gel. The
organic solution is concentrated under reduced pressure to give a light brown oil.
The material is distilled (bulb to bulb, 0.002 mm). A low boiling fraction (bp 58-70°C)
is collected; the distillation is stopped and the low boiling material is rinsed out
of the receiver bulb. The distillation is resumed (bulb-to-bulb, bp 84°C at 0.002 mm) to give a yellow solid that melts at
room temperature to give 9.72 g
(90%) of the desired product
as a pale yellow oil (Note 13).
2. Notes
1.
Pd
2(dba)
3,
palladium
acetate, and (±)-BINAP were purchased from
Strem Chemical Company
and used without further purification.
(The checkers recrystallized Pd(OAc)
2 from
benzene
prior to use. When it was used as obtained from the supplier, the reaction did not
go to completion.)
2.
Sodium tert-butoxide
is purchased from Aldrich Chemical Company, Inc.
,
and used without further purification. The bulk of this material is stored under nitrogen
in a Vacuum Atmospheres Glovebox. Small portions (10-15 g) were removed from the glovebox
in glass vials and weighed in the air. The checkers stored
sodium tert-butoxide,
purchased in 5-g bottles, in a desiccator and weighed it out in air.
3.
Toluene is distilled under
nitrogen
from molten
sodium.
4.
4-Bromo-3-methylanisole
is purchased from Aldrich Chemical Company, Inc.
,
and used without further purification.
5.
All amines were purchased from Aldrich Chemical Company, Inc.,
and distilled under
argon from
calcium hydride
(CaH
2).
6.
Reaction times were considerably longer when run on a large scale
than the previously published reaction times. This may be due to the lower internal
temperature of the large scale reactions (for example, a reaction run on a 50-mmol
scale in a 70°C oil bath was found to have an internal temperature of only 60°C).
Longer reaction times in reactions that used
cesium carbonate
as a base may also be due in part to differences in stirring rate.
7.
Analytical data for this compound are are as follows:
1H NMR (300 MHz, CDCl
3)
δ: 0.90 (t, 3 H, J = 6.6), 1.30-1.67 (m, 8 H), 2.13
(s, 3 H), 3.09 (t, 2 H, J = 7.2), 3.12 (s, br, 1 H),
3.74 (s, 3 H), 6.53-6.57 (m, 1 H), 6.69-6.71 (m, 2
H)
;
13C
NMR (75 MHz, CDCl
3) δ: 14.0, 17.6, 22.6,
26.9, 29.6, 31.6, 44.7, 55.6,
110.7, 111.5, 116.8, 123.5, 140.7,
151.4
; IR (neat) cm
−1:
3414, 2928, 1514, 1225, 1051.
Anal. Calcd for C
14H
23NO: C, 75.97; H, 10.47. Found: C, 75.93;
H, 10.45.
8.
THF was distilled under argon from
sodium benzophenone
ketyl.
9.
4-Chlorophenyl trifluoromethanesulfonate
2a is prepared according to the procedure
of stille
2b,c as follows: A
500-mL,
oven-dried, round-bottomed flask equipped with a
Teflon stirbar
and a
rubber septum is allowed to cool to room temperature
under an argon purge. The septum is removed and the flask was charged with
4-chlorophenol (16.1 g, 125 mmol)
(Note 14). The septum is again placed on the flask, the flask
is purged with argon, and
pyridine (125 mL)
(Note 14) is added via syringe. The mixture is cooled to 0°C
with stirring in an ice-water bath and
trifluoromethanesulfonic
anhydride (163 mmol)
(Note 14) is added
dropwise via syringe. After the addition is complete, the mixture is stirred at 0°C
for 15 min, then allowed to warm to room temperature and stirred for 22 hr. The mixture
is diluted with hexanes/ether (300 mL, 1/1 v/v) and transferred to a separatory funnel.
The mixture is washed with
aqueous hydrochloric
acid (HCl) (1 M, 2 × 200 mL), and
brine (200 mL), and the layers are
separated. The organic layer is dried over anhydrous magnesium sulfate, filtered,
and concentrated under reduced pressure. The crude material is Kugelrohr-distilled
under vacuum (
bp 90°C at water
aspirator vacuum) to afford
31 g
(
95%) of the title compound
as a colorless oil. This material is passed through a short plug of silica gel prior
to use in palladium-catalyzed amination reactions.
10.
Cesium carbonate
was obtained from Chemetal
and used without further
purification. The bulk of this material was stored under
nitrogen
in a Vacuum Atmospheres Glovebox. Small portions (~50 g) were removed from the glovebox
in glass bottles and weighed in the air. (The checkers stored the
cesium
carbonate in a
desiccator under argon, and weighed
it in air.)
11.
The formation of a side product,
N,N'-dimethyl-N,N'-diphenyl-1,4-phenylenediamine,
was observed if the internal reaction temperature exceeded 65°C.
12.
This reaction was run three times on a 50-mmol scale. Two of
the three reactions were complete in <30 hr. The third reaction was run with a
batch of triflate that had not been passed through silica as described above
(Note 9); this reaction took 45 hr to proceed to completion. A second
sample of the triflate was passed through a plug of silica; the reaction was repeated
(on a 40-mmol scale) and proceeded to completion in 26 hr.
13.
Analytical data for this compound are as follows:
1H NMR (300 MHz), CDCl
3) δ:
3.28 (s, 3 H), 6.88-7.32 (m, 9 H)
;
13C NMR (75 MHz, CDCl
3)
δ: 40.3, 120.5, 121.4, 122.2,
125.5, 129.0, 129.3, 147.6, 148.6
;
IR (neat) cm
−1: 3061,
2943, 1601, 1452, 1156, 817
;
GC/MS (m/z) 219.
Anal. Calcd for C
13H
12ClN: C, 71.87; H, 5.57. Found: C, 71.95;
H, 5.81.
14.
4-Chlorophenol, trifluoromethanesulfonic anhydride, and anhydrous
pyridine were purchased from Aldrich Chemical Company, Inc., and used without further
purification.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Aniline derivatives are frequently used in many areas of chemistry, including pharmaceuticals,
3a agrochemicals,
3b photography,
3c
pigments,
3d xerography,
3e and materials.
3f Classic
methods for the construction of aryl carbon-nitrogen bonds usually require harsh reaction
conditions and/or activated substrates, and are often inefficient or unreliable.
4
5a
The palladium-catalyzed
amination of aryl halides has recently emerged as a powerful alternative to other
methods for the synthesis of aryl amines.
5 This method
allows for the cross-coupling of aryl halides and triflates with amines in the presence
of a stoichiometric amount of a base and catalytic amounts of palladium complexes
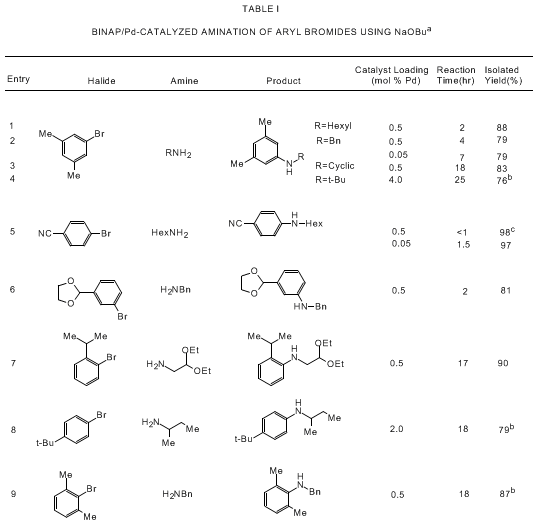
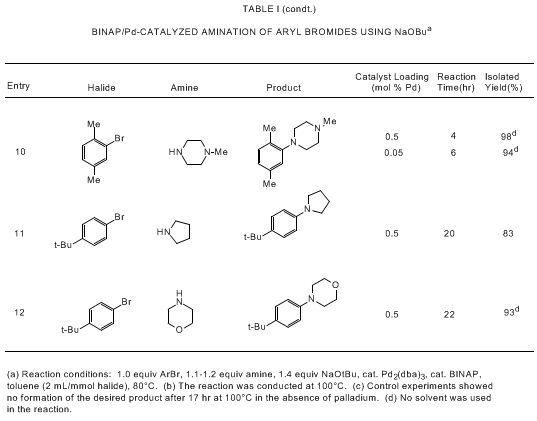
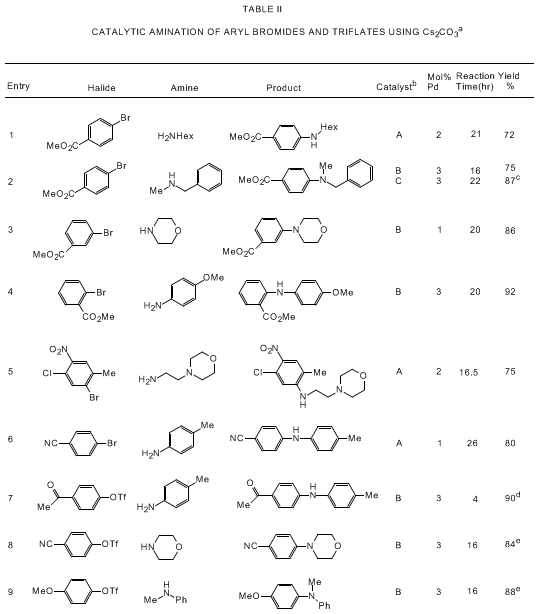
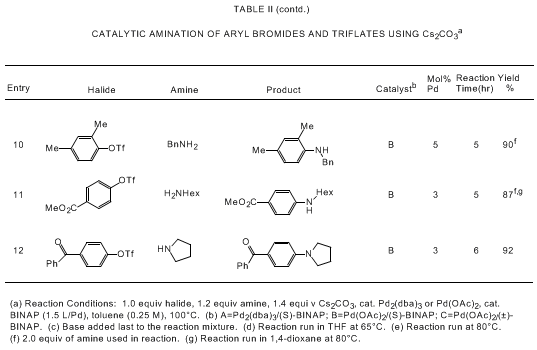
bearing tertiary phosphine ligands. As shown in Tables I and II, the Pd/BINAP catalyst
is highly effective for reactions of aryl bromides and triflates with primary and
cyclic secondary amines. Use of the strong base NaOtBu allows for rapid reactions
with low levels of the palladium catalyst (usually 0.05-0.5 mol % Pd) while a high
degree of functional group tolerance is observed if Cs
2CO
3 is
used as base.
5a,d,e,i Catalytic aminations
of aryl triflates are most effective with Cs
2CO
3;
5a,e cleavage of the triflate moiety is observed in
the presence of NaOtBu.
5a,e
Catalysts based on bulky, electron-rich phosphine ligands have recently been developed
that are effective for aminations of aryl chlorides, as well as aryl bromides and
triflates.
5f,g,j, These catalysts also
promote room temperature coupling reaction.
5f,j
The examples described here demonstrate that a variety of aryl halides or triflates
with differing substitution patterns, electronic properties, and functional groups
can be coupled with primary and secondary amines in high yield. The reactions are
only modestly air sensitive, require no special equipment or techniques, and are amenable
to large scale synthesis.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
N-Methyl-N-(4-chlorophenyl)aniline:
Benzenamine,
4-chloro-N-methyl-N-phenyl- (13); (174307-94-9)
Tris(dibenzylideneacetone)dipalladium (0):
Palladium,
tris(1,5-diphenyl-1,4-pentadien-3-one)di- (9); (52409-22-0)
rac-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl:
rac-BINAP:
Phosphine, [1,1'-binaphthalene]-2,2'-diylbis{diphenyl-
(11); (98327-87-8)
4-Bromo-3-methylanisole:
Anisole, 4-bromo-3-methyl-
(8);
Benzene, 1-bromo-4-methoxy-2-methyl- (9); (27060-75-9)
Hexylamine (8);
1-Hexanamine (9);
(111-26-2)
Palladium acetate:
Acetic acid, palladium(2+)
salt (9); (3375-31-3)
4-Chlorophenyl trifluoromethanesulfonate:
Methanesulfonic
acid, trifluoro-, p-chlorophenyl ester (8);
Methanesulfonic acid,
trifluoro-, 4-chlorophenyl ester (9); (29540-84-9)
N-Methylaniline:
Aniline, N-methyl-
(8);
Benzenamine, N-methyl- (9); (100-61-8)
Cesium carbonate:
Carbonic acid, dicesium
salt (9); (534-17-8)
4-Chlorophenol: TOXIC:
Phenol, p-chloro-
(8);
Phenol, 4-chloro- (9); (106-48-9)
Pyridine (8,9); (110-86-1)
Trifluoromethanesulfonic anhydride:
Methanesulfonic
acid, trifluoro-, anhydride (8,9); (358-23-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved