Org. Synth. 1990, 69, 106
DOI: 10.15227/orgsyn.069.0106
HYDROMAGNESIATION REACTION OF PROPARGYLIC ALCOHOLS: (E)-3-PENTYL-2-NONENE-1,4-DIOL FROM 2-OCTYN-1-OL
Submitted by Fumie Sato and Yuichi Kobayashi
1.
Checked by Zuliang Zhou and Ekkehard Winterfeldt.
1. Procedure
A. The Grignard reagent 2. A dry, 500-mL, three-necked, round-bottomed flask containing a magnetic stirring bar is equipped with a 100-mL pressure-equalizing dropping funnel, a glass stopper, and a two-way stopcock to which is attached a T-piece connected at one end to a supply of nitrogen, and at the other to an oil bubbler (Note 1). The flask is charged with a solution of isobutylmagnesium chloride in ether (320 mL, 0.75 M, 240 mmol) (Note 2) and (Note 3) and immersed in an ice–water bath. Titanocene dichloride (1.3 g, 5.2 mmol) (Note 4) is added at once and the resulting solution is allowed to stir at 0°C for 10 min. A solution of 2-octyn-1-ol (1) (13.2 g, 105 mmol) (Note 5) in ether (30 mL) (Note 3) is placed in the dropping funnel and added dropwise to the flask over 20 min at 0°C. The solution is stirred at room temperature for 4 hr to complete the reaction, affording Grignard reagent 2 (Note 6),(Note 7),(Note 8).
B. (E)-3-Pentyl-2-nonene-1,4-diol (4). Half the amount of the Grignard reagent 2 prepared according to Procedure A is diluted with ether (160 mL) and cooled in an ice–water bath to 0°C. A solution of hexanal (9.25 g, 92.5 mmol) (Note 9) dissolved in ether (30 mL) (Note 3) is added through the dropping funnel over 30 min with efficient stirring. After the addition is complete, the solution is stirred at 0–5°C for 2 hr and then poured into saturated ammonium chloride solution (300 mL). The mixture is stirred at 0°C for 1 hr. The resulting precipitate is removed by filtration through a pad of Celite (70 × 24 mm) under reduced pressure and the precipitate is washed with ethyl acetate (100 mL). The organic layer is separated and the aqueous phase is extracted with ethyl acetate (150 mL). The combined organic layers are dried over magnesium sulfate and concentrated under reduced pressure to leave an oil that is purified by column chromatography on silica gel (Note 10) and (Note 11) to afford 4 (7.0–7.4 g, 59–62% yield) (Note 12).
2. Notes
1.
Reactions A and B are carried out under a
nitrogen atmosphere. The submitters used
argon.
2.
A solution of
isobutylmagnesium chloride in
ether was prepared using
isobutyl chloride (13.8 g, 150 mmol),
magnesium turnings (4.05 g, 165 mg-atom), and
ether (175 mL) according to the procedure for the preparation of
sec-butylmagnesium chloride reported by Gilman and Kirby.
2 The checkers found that this solution contained about
145 mmol of isobutylmagnesium chloride.
3.
Ether and
tetrahydrofuran were distilled from
benzophenone ketyl under an
argon atmosphere.
4.
Titanocene dichloride was purchased from Aldrich Chemical Company, Inc., and used without further purification.
5.
Alcohol
1 was prepared according to the procedure of Rickards and Weiler
3 and distilled under reduced pressure (102–108°C at 15 mm) before use. This material is also commercially available from Farchan Laboratories, Inc. The checkers obtained it from Lancaster Chemical Co.
6.
The checkers found that Grignard reagent
2 could not be obtained quantitatively although all of the
isobutylmagnesium chloride had reacted. The end point of the reaction was determined by TLC analysis of a small amount of reaction mixture after hydrolysis.
2-Octyn-1-ol (
1) and
(Z)-2-octen-1-ol (3), have
Rf values of 0.53 and 0.47, respectively (using Silica Gel 60 F
254 precoated TLC aluminum sheets and
benzene–ethyl acetate (1 : 1) as developing agent). If the reaction is not complete, an additional amount of
titanocene dichloride should be added to the solution, which is cooled again to 0°C before the addition.
7.
The submitters report that, if this solution is poured into
1 N hydrochloric acid and ice and worked up in the usual manner, (
Z)-2-octen-1-ol (
3) may be obtained in
86% yield by distillation, bp
97–101°C (12 mm). This product has the following spectra: IR (neat) cm
−1: 3290, 1457, 1014;
1H NMR (90 MHz, CCl
4, (CH
3)
4Si, D
2O) δ: 0.88 (t, 3 H,
J = 6), 1.05–1.57 (m, 6 H), 1.85–2.22 (m, 2 H), 4.03 (d, 2 H,
J = 5), 5.27–5.65 (m, 2 H).
8.
The submitters report that, if this solution is concentrated, then dissolved in
tetrahydrofuran and treated with
methyl iodide,
(Z)-3-methyl-2-octen-1-ol (5) may be obtained after silica gel chromatography using
hexane–ether as eluent. The product has the following spectra: IR (neat) cm
−1: 3305, 1447, 1000;
1H NMR (90 MHz, CCl
4, (CH
3)
4Si, D
2O) δ: 0.88 (t, 3 H,
J = 6), 1.1–1.6 (m, 6 H), 1.68 (s, 3 H), 1.9–2.2 (m, 2 H), 3.99 (d, 2 H,
J = 6), 5.29 (t, 1 H,
J = 6).
9.
Hexanal was used as supplied by Tokyo Kasei Kogyo Co., Ltd. (Japan). The checkers obtained it from Aldrich Chemical Company, Inc.
10.
Silica gel (100–200 mesh) was purchased from Wako Pure Chemical Industries, LTD (Japan).
11.
A silica gel column (225 g, 60 × 210 mm) is used with a mixture of
benzene and
ethyl acetate as an eluent [
Rf value (
benzene–
ethyl acetate = 1 : 1):
3, 0.47;
4, 0.23]. Distillation of product
4 under reduced pressure caused partial decomposition (bp
120–155°C/0.25 mm).
12.
Diol
4 has the following spectra: IR (neat) cm
−1: 3300, 1468, 1020;
1H NMR (90 MHz, CDCl
3, (CH
3)
4Si, D
2O) δ: 0.89 (t, 6 H,
J = 6), 1.1–1.7 (m, 14 H), 1.9–2.2 (m, 4 H), 4.02 (m, 1 H), 4.20 (d, 2 H,
J = 6), 5.63 (t, 1 H,
J = 6). With D
2O exchange there is a change in the range of δ 1.9–2.2 (m, 2 H).
3. Discussion
In 1962 Cooper and Finkbeiner reported the
titanium chloride (TiCl
4)-catalyzed exchange reaction of an alkyl Grignard reagent (RMgX) having β-hydrogen(s) with olefins (Eq. 1).
4 In this reaction, RMgX can be formally regarded as a source of HMgX that adds to the olefins, and hence this reaction is known as the hydromagnesiation reaction.
Since then, hydromagnesiation of other unsaturated hydrocarbons such as conjugated dienes and acetylenes has been investigated intensively.
5,6 Hydromagnesiation of 2-alkyl substituted 1,3-butadienes has been shown to proceed regiospecifically by using
Cp2TiCl2 as a catalyst to afford the corresponding allylic Grignard reagent shown in Eq. 2 quantitatively.
7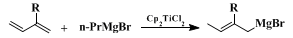
Cp2TiCl2-catalyzed hydromagnesiation of acetylenes with isobutyl Grignard reagents has also been shown to provide a convenient and practical method for preparation of various vinyl Grignard reagents. The acetylenes so far examined include 1,2-dialkylacetylenes
6 (Eq. 3),
1-(trimethylsilyl)acetylenes 7 (Eq. 4),
8 propargyl alcohols
8 (Eq. 5),
9 and
3-(trimethylsilyl)propargyl alcohol (9) (Eq. 6).
10 Although the reaction occurs with low regioselectivity for unsymmetric dialkylacetylenes
6, high regioselectivity is attained in the case of
7, 8, and
9. Acetylenes
6, 7, and
8 afford the corresponding vinylmagnesium halides in which HMgX adds in a
syn pathway to the triple bond, while
9 affords the
anti-addition product. In the latter case, hydromagnesiation follows the
syn pathway to yield the corresponding (
Z)-alkenyl Grignard reagent first, which, however, isomerizes rapidly under the reaction conditions to the (
E)-alkenyl Grignard reagent. Since the hydromagnesiation reaction of
7, 8, and
9 proceeds highly regio- and stereo-selectively, the reaction has become a powerful synthetic tool for utilization in organic syntheses. Some of the examples are given in Table I.
TABLE I
|
|
Starting Acetylene
|
i-BuMgX X
|
Vinylmagnesium Halide
|
Electrophile
|
Product
|
Yield %
|
Ref.
|
|
|
|
Br
|
|
I2
|
|
70
|
8
|
|
|
Br
|
|
|
|
86
|
11
|
|
|
Cl
|
|
I2
|
|
78-86
|
9
|
|
|
Cl
|
|
Mel
|
|
95
|
9
|
|
|
Br
|
|
|
|
83
|
12
|
|
|
Br
|
|
EtCN
|
|
86
|
13
|
|
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
benzophenone ketyl
Cp2TiCl2
1-(trimethylsilyl)acetylenes
Br
Cl
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
ethyl acetate (141-78-6)
ether (60-29-7)
ammonium chloride (12125-02-9)
magnesium turnings (7439-95-4)
nitrogen (7727-37-9)
isobutyl chloride (513-36-0)
Methyl iodide (74-88-4)
magnesium sulfate (7487-88-9)
Tetrahydrofuran (109-99-9)
hexane (110-54-3)
titanium chloride (7550-45-0)
argon (7440-37-1)
3-(trimethylsilyl)propargyl alcohol (5272-36-6)
Hexanal (66-25-1)
2-OCTYN-1-OL (20739-58-6)
isobutylmagnesium chloride (5674-02-2)
Titanocene dichloride (1271-19-8)
(E)-3-Pentyl-2-nonene-1,4-diol (138149-15-2)
sec-butylmagnesium chloride (15366-08-2)
(Z)-2-octen-1-ol (26001-58-1)
(Z)-3-methyl-2-octen-1-ol (30804-78-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved