Org. Synth. 2023, 100, 48-60
DOI: 10.15227/orgsyn.100.0048
Synthesis of NH-Sulfoximines from Sulfides Using Ammonium Carbamate and (Diacetoxyiodo)benzene to Transfer NH and O
Submitted by Arianna Tota, Mauro Spennacchio, Edward L. Briggs, Tsz-Kan Ma, Zhenhao Zhong, Leonardo Degennaro, James A. Bull,* and Renzo Luisi*
Checked by Ellie Plachinski and Tehshik Yoon
1. Procedure (Note 1)
(4-Bromophenyl)(imino)(methyl)-λ6-sulfanone (2). In air, a 250 mL one-necked, round-bottomed flask (24/40), equipped with a Teflon coated oval-shaped magnetic stirrer bar (32 mm × 15 mm) is suspended in a water bath at 25 °C and charged with MeOH (70 mL) (Note 2). Stirring of the solvent 500 rpm is initiated. (4-Bromophenyl)(methyl)sulfane (1) (7.11 g, 35 mmol, 1.0 equiv) (Note 3) and ammonium carbamate (5.47 g, 70 mmol, 2.0 equiv) (Note 4) are added sequentially as single portions via a powder funnel. (Diacetoxyiodo)benzene (28.18 g, 87.5 mmol, 2.5 equiv) (Note 5) is then added in four roughly equal portions (over a period of 5 min), allowing for controlled decarboxylation (Figure 1a). The reaction mixture is stirred at 25 °C (ambient temperature) for 3 h, during which time the reaction mixture turns from colorless to pale yellow (Figure 1b). The reaction progress can be monitored by TLC (Note 6).
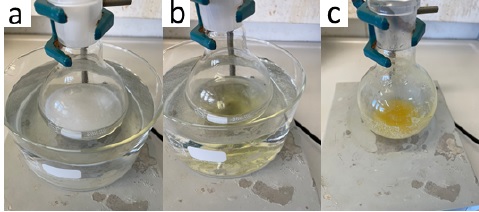
Figure 1. a) Decarboxylation of ammonium carbamate; b) Reaction mixture after 3 h; c) Reaction mixture after removal of methanol (photos provided by checkers)
The organic solvent is evaporated under reduced pressure (25 ºC, 35 mmHg) to give a yellow slurry (Figure 1c), which is diluted with EtOAc (150 mL) (Note 7) and saturated aqueous NaHCO3 (100 mL) (Note 8), and then stirred for 30 min to neutralize the acetic acid formed during the reaction. The resulting biphasic solution is transferred to a 1 L separatory funnel, rinsing the flask with EtOAc (50 mL). The layers are separated, and the organic layer is washed with distilled H2O (100 mL) to remove residual ammonium carbamate. The layers are separated. The combined aqueous fractions are extracted with EtOAc (3 x 100 mL) and subjected to the subsequent workup procedure to yield additional product. The organic fraction is extracted with aqueous 1M HCl (3 x 100 mL) (Figure 2a). The acidic aqueous layers (pH ≈ 1) are combined, transferred to a clean separatory funnel, and washed with CH2Cl2 (3 x 100 mL) (Note 9). The aqueous fraction is then basified to pH ≈ 12 with aqueous 2M NaOH (200 mL) and extracted with CH2Cl2 (3 x 100 mL). The combined organic fractions are dried over anhydrous Na2SO4 (ca. 20 g) (Note 10). The solution is filtered through a 7 cm glass funnel plugged with cotton wool and the Na2SO4 is washed with CH2Cl2 (50 mL) into a 500 mL round-bottomed flask (Figure 2b). The organic solvent is evaporated under reduced pressure (25 °C, 250 mmHg), and the flask is then placed under high vacuum (21 °C, 0.11 mmHg) for 18 h to remove any residual solvent to yield (4-bromophenyl)(imino)(methyl)-λ6-sulfanone (2) as a white solid (5.22 g, 63%, 98% purity) (Figure 2c) (Notes 11, 12 and 13).
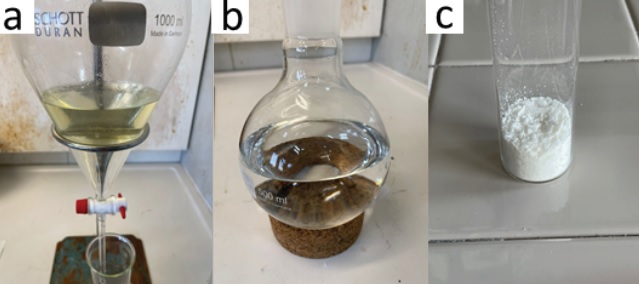
Figure 2. a) Extraction using aqueous HCl (1M); b) Product in CH2Cl2 after work up; c) Final compound 2 (photos provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
methanol,
(4-Bromophenyl)(methyl)sulfane,
ammonium carbamate,
(diacetoxyiodo)-benzene,
ethyl acetate, aqueous
sodium bicarbonate,
,aqueous 1M
hydrochloric acid,
dichloromethane, aqueous 2M
sodium hydroxide, anhydrous
sodium sulfate,
hexanes,
potassium permanganate,
CDCl3, and
1,3,5-trimethoxybenzene.
2. HPLC grade
methanol (99.8%) was purchased from Fisher Scientific and used as received
3.
(4-Bromophenyl)(methyl)sulfane 1 (97%) was purchased from Sigma-Aldrich and used as received.
4.
Ammonium carbamate (99%) was purchased from Sigma-Aldrich and used as received.
5.
(Diacetoxyiodo)benzene (98%) was purchased from Fluorochem and used as received.
6. Reaction progress can be monitored by silica gel TLC analysis performed with 100%
hexanes or 40%
EtOAc in
hexanes using
KMnO4 as a stain. TLC Silica gel 60 F254 glass backed plates were purchased from Merck, Inc. The starting material
1 has a R
f = 0.8 and the product
2 has a R
f = 0 using
hexanes as eluent (Figure 3a). The starting material
1 has a R
f = 0.95 and the product
2 has a R
f = 0.10 using 40%
EtOAc in
hexanes as eluent (Figure 3b).
Figure 3. (a) TLC analysis of reaction using hexanes as the eluent; (b) TLC analsysis using 40% EtOAc in hexanes as eluent (photos provided by checkers)
7.
Ethyl acetate (99.5%) was purchased from Sigma-Aldrich and used as received.
8.
NaHCO3 (99%) was purchased from Sigma-Aldrich and used as received.
9.
Dichloromethane (99.9%) was purchased from Sigma-Aldrich and used as received.
10. Anhydrous
sodium sulfate (99%) was purchased from Fisher Scientific and used as received.
11. A second run of the reaction on similar scale provided 5.00 g (62%) of the desired product.
12. The purity of the compound was calculated by quantitative
1H NMR
pdf with a relaxation delay of 30 seconds using 21 mg of
1,3,5-trimethoxybenzene (purity 99%) and 31 mg of compound
2.
13. Analytical data for
(4-bromophenyl)(imino)(methyl)-λ6-sulfanone (
2): mp = 89-90 °C.
1H NMR
pdf (500 MHz,
CDCl3) δ: 7.89-7.87 (m, 2H), 7.71-7.69 (m, 2H), 3.10 (s, 3H), 2.72 (s, 1H).
13C NMR
pdf (101 MHz,
CDCl3) δ: 142.7, 132.6, 129.4, 128.3, 46.2. IR (film) 3303, 3274, 3083, 3009, 2928, 1570, 1468, 1215, 1090, 1063, 1020 cm
-1.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Sulfoximines are emerging motifs in the life sciences and have shown application in asymmetric synthesis as chiral auxiliaries, ligands and catalysts.
2 Their Lewis basic character has also made them interesting directing groups for C-H functionalization.
3 Their most promising applications are in the pharmaceutical and agrochemical industries as bioisosteres for sulfones and sulfonamides.
4 Recent years have seen broad uptake in medicinal chemistry and several examples of sulfoximines in clinical candidates.
4b,5 Until relatively recently, uptake in the life sciences had been limited by the lack of safe and amenable synthetic methods, which have seen significant recent development.
6,7 In 2017 we reported an operationally simple metal-free protocol for the straightforward preparation of NH-sulfoximines from sulfides using
ammonium carbamate as a convenient source of ammonia and
(diacetoxyiodo)benzene as the oxidant.
8 This protocol was applied by us (and others) for the preparation of NH-sulfoximines and for the
NH- and
O-transfer to sulfenamides to access sulfonimidamides.
8,9,10From a mechanistic point of view, it was demonstrated that the process involves the initial condensation of ammonia with the hypervalent iodine reagent leading to an iminoiodinane which undergoes oxidation to form a highly reactive iodonitrene. The iodonitrene reacts directly with the sulfide to form the corresponding sulfilimine that reacts with the O-donor (the alcoholic solvent or AcOH) ending up with the formation of a sulfanenitrile that converts into the corresponding NH-sulfoximine. For mechanistic evidence see the original report,
11 as well as related reports on sulfides
8,9a and sulfenamides.
10 The methodology has been widely employed for the direct one-pot
NH and
O-transfer to challenging substrates such as fluorinated sulfides,
12 thioglycosides,
13 and even to thiols.
14 In conclusion, we expect this metal-free protocol from sulfides to be widely applicable, with the demonstrated compound presenting an attractive building block for further derivatization.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
4-(Bromophenyl)(methyl)sulfane: Benzene, 1-bromo-4-[methylsulfanyl]-; (1) (104-95-0)
Ammonium carbamate: Carbamic acid, ammonium salt (1:1); (1111-78-0)
(Diacetoxyiodo)benzene: Iodine, bis(acetato-κO)phenyl-; (3240-34-4)
(4-Bromophenyl)(imino)(methyl)-λ6-sulfanone: Sulfoximine, (4-bromophenyl)-S-methyl-; (2) (2258639-09-5)
Methanol; (67-56-1)

|
Arianna Tota obtained the M.Sci. (summa cum laude) in Chemistry and Pharmaceutical Technology at the University of Bari (Italy) in 2015. In 2020, she obtained the Ph.D. in Chemical and Molecular Sciences under the supervision of Prof. Renzo Luisi. Her research activity is focused on the electrophilic nitrogen transfer to sulfur and the chemistry of nitrogen-bearing compounds. In 2019, she has been a visiting scholar at the Department of Synthetic Chemistry and Biological Chemistry, Kyoto University (Japan), working in the group of Prof. Aiichiro Nagaki. During this time, she was involved in the field of flow microreactor technology applied to organometallic chemistry. |

|
Mauro Spennacchio obtained the master degree (summa cum laude) in Pharmacy at the University of Bari (Italy) in 2019. He is currently at the third year of his Ph.D. in Pharmaceutical Science under the supervision of Prof. Renzo Luisi and Prof. Leonardo Degennaro. His research activity focuses on the development of new fluorination strategies exploiting flow microreactor technology and the electrophilic nitrogen transfer to sulfur. |

|
Edward Briggs obtained his MChem Chemistry with six-month placement in 2017 from the University of Southampton. During this time, he worked with Professor Bruno Linclau on the fluorination of glucose and galactose derivatives, as well as undertaking a placement at Vertex Pharmaceuticals where he worked simultaneously on bespoke reagent synthesis and the lead medicinal chemistry project. Edward started his Ph.D. with Dr James Bull at Imperial College London in October 2017 looking at novel synthetic methods to access sulfoximines and sulfonimidamides. |

|
Tsz-Kan Ma received his M.Sci. in Chemistry with Medicinal Chemistry at Imperial College, where he continued his Ph.D. studies in biomimetic total synthesis of meroterpenoid natural products under the supervision of Prof. Anthony G. M. Barrett. He then moved to UCL as a research fellow in organic electrosynthetic chemistry, working with Dr. Jonathan D. Wilden to develop new electrosynthetic protocols. He is now back at Imperial College London, working with Dr. James Bull as a research associate in synthetic chemistry, focus on developing new synthetic protocols for transition metal catalyzed C-H activation of heterocyclic compounds. |

|
Zhenhao Zhong received his B.Sc degree in chemistry jointly from Nanjing Tech University and the University of Sheffield in 2019. Then he obtained his MRes degree in advanced molecular synthesis at Imperial College London. Zhenhao continued his Ph.D. studies at the same institution under the supervision of Dr. James Bull from October 2020, focusing on developing novel synthetic methods to synthesize sulfoximines and sulfonimidamides. |

|
Leonardo Degennaro obtained the master's degree in Chemistry and Pharmaceutical Technology in 1999 and the Ph.D. in Applied Chemical and Enzymatic Synthesis in 2003. In 2002 he was "visiting scholar" at the University of Groningen under the supervision of Prof. B. L. Feringa. In 2006 he was appointed assistant professor in Organic Chemistry at the Department of Pharmacy of University of Bari. In 2011 he was "visiting assistant professor" at the University of Kyoto working in the group of Prof. J.-i. Yoshida. The research activity is aimed at developing new stereocontrolled synthesis by using small heterocycles and organometallic species, and microreactor technology |

|
Dr. James Bull is a University Research Fellow at Imperial College London. His research focuses on the development of synthetic and catalytic methods to access medicinally relevant structural motifs and heterocycles. He obtained his MSci degree from the University of Cambridge, then spent a year at GlaxoSmithKline. He returned to University of Cambridge for his Ph.D. with Professor Steven Ley. In 2007 he joined Université de Montréal as a postdoc with Professor André Charette. He started a Ramsay Memorial Fellowship at Imperial College in 2009, an EPSRC Career Acceleration Fellowship in 2011, and in 2016 was awarded a Royal Society URF. |

|
Renzo Luisi is full professor of Organic Chemistry at the University of Bari (Italy). The research activity focuses on the chemistry of hetero-substituted organolithiums, the development of new synthetic methodologies, and the use of flow technology. He obtained the Ph.D. in 2000 under the guidance of Professor Saverio Florio. He has been visiting student at the Roger Adams Lab at Urbana Champaign in the group of Prof. Peter Beak, and visiting professor at the University of Manchester in the group of Jonathan Clayden. He is RSC fellow and recipient of the 2014 CINMPIS award Innovation in Organic Synthesis. |

|
Ellie Plachinski obtained a B.S. in chemistry in 2020 from the Massachusetts Institute of Technology, where she conducted research in the laboratory of Prof. Alison Wendlandt. She is currently pursuing her Ph.D. in the Yoon Group in the Department of Chemistry at the University of Wisconsin-Madison. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved