Org. Synth. 2003, 80, 1
DOI: 10.15227/orgsyn.080.0001
SYNTHESIS OF 1,2:4,5-DI-O-ISOPROPYLIDENE-D-erythro-2,3-HEXODIULO-2,6-PYRANOSE. A HIGHLY ENANTIOSELECTIVE KETONE CATALYST FOR EPOXIDATION
[[β-D-erythro-2,3-Hexodiulo-2,6-pyranose, 1,2:4,5-bis-O-(1-methylethylidene)-]]
Submitted by Yong Tu, Michael Frohn, Zhi-Xian Wang, and Yian Shi.
1.
Checked by Jason M. Diffendal and Rick L. Danheiser.
1. Procedure
A.
1,2:4,5-Di-O-isopropylidene-β-D-fructopyranose(
1).
2 D-Fructose (18.0 g, 100 mmol) (Note 1) and
2,2-dimethoxypropane (7.4 mL, 60 mmol) (Note 1) are added to
350 mL of acetone (Note 1) in a
1-L, round-bottomed flask equipped with a
Teflon-coated magnetic stir bar. The flask is cooled in an
ice bath for 15 min, then
4.3 mL of 70% perchloric acid (Note 2) is added in one portion. The resulting suspension is stirred for 6 hr at 0°C
(Note 3). Concentrated
ammonium hydroxide (4.8 mL) is then added to neutralize the acid and the solvent is removed by rotary evaporation at 25°C to give a white solid. This solid is dissolved in
200 mL of dichloromethane (CH
2Cl
2) and washed with two
50-mL portions of saturated sodium chloride solution, dried over
sodium sulfate (Na
2SO
4), filtered, and concentrated by rotary evaporation (25°C) until the total volume is about 40 mL
(Note 4). Boiling
hexane (100 mL) is then added
(Note 5) and the flask is allowed to cool to room temperature, during which time the bulk of the product crystallizes out of solution. Additional product crystallizes upon cooling to −25°C for 4 hr. Isolation of the solid by vacuum filtration and careful washing with three
25-mL portions of cold (−25°C) hexane gives
13.4-13.6 g (
51-52%) of the title alcohol as fine white needles
(Note 6).
B.
1,2:4,5-Di-O-isopropylidene-D-erythro-2,3-hexodiulo-2,6-pyranose (
2).
2 A
500-mL, round-bottomed flask equipped with a
4.5-cm, egg-shaped Teflon-coated magnetic stir bar is charged with
130 mL of CH2Cl2 (Note 1), the
alcohol prepared in Step A (10.4 g, 40.0 mmol), and 15 g of freshly powdered 3 Å molecular sieves
(Note 7).
Pyridinium chlorochromate (21.5 g, 100 mmol) (Note 1) is added portionwise over 10 min and the resulting mixture is stirred at room temperature for 15 hr
(Note 8).
Ether (200 mL) is added slowly with vigorous stirring and the solution is filtered under vacuum through a pad of 35 g of Celite
(Note 9). The solids remaining in the reaction flask are transferred to the Celite pad by scraping with a
spatula and washing with three
50-mL portions of ether. The resulting cloudy brown filtrate is concentrated by rotary evaporation at room temperature to give a brown solid. To this solid is added
25 mL of 1:1 ether:hexane and the solids are scraped with a spatula. The mixture is then poured onto 60 g of Whatman 60 Å (230-400 mesh) silica gel packed in a 4-cm diameter chromatography column and the liquid is adsorbed onto the silica gel by gravity
(Note 10). The material remaining in the flask is further washed with
1:1 ether:hexane and transferred onto the silica gel; this process is repeated until all the material has been loaded onto the silica gel. The ketone is eluted using
500 mL of 1:1 ether:hexane and the eluent is concentrated by rotary evaporation to afford the crude ketone as a white solid. This material is dissolved in
40-45 mL of boiling hexane. Upon cooling the solution to room temperature, the ketone begins to crystallize. The flask is then cooled to −25°C for 2 hr. The resulting solids are collected by filtration, washed with three
25-mL portions of cold (−25°C) hexane, and dried to afford
8.84-9.08 g, (
86-88%) of the ketone as a white solid
(Note 11).
2. Notes
1.
D-Fructose (98%), 2,2-dimethoxypropane (98%), and pyridinium chlorochromate (98%) were obtained from Aldrich Chemical Company, Inc. and used as received.
ACS grade acetone and dichloromethane were purchased from Fisher Scientific and used as received.
2.
Perchloric acid (70%) was obtained from J. T. Baker Company. Reaction of
70% perchloric acid with organic materials can lead to fires and explosions, and anhydrous HClO
4 is potentially explosive. Although no incidents occurred in the experience of the submitters, care should be taken in handling this compound.
3.
The suspension turns into a clear, colorless solution after 1-2 hr. The title compound is the kinetic product of the reaction, and can readily isomerize to
2,3:4,5-di-O-isopropylidene-β-D-fructopyranose (the thermodynamic product; see ref.
2c). Control of the reaction time is important to minimize the formation of the thermodynamic product.
4.
The solvent volume can vary slightly without much effect on the yield of the recrystallization step.
5.
The white, crystalline product begins to precipitate in the first 5 min after the addition of
hexane.
6.
The product exhibits the following physical and spectral properties:
mp 118.5-119.5°C; IR (KBr) cm
−1: 3547;
1H NMR (500 MHz, CDCl
3) δ: 1.37 (s, 3 H), 1.44 (s, 3 H), 1.52 (s, 3 H), 1.54 (s, 3 H), 1.99 (d, 1 H, J = 8.1), 3.67 (dd, 1 H, J = 8.1, 6.8), 3.98 (d, 1 H, J = 9.0), 4.01 (dd, 1 H, J = 13.2, 0.9), 4.12 (dd, 1 H, J = 13.2, 2.7), 4.13 (dd, 1 H, J = 6.8, 5.7), 4.19 (d, 1 H, J = 9.0), 4.22 (ddd, 1 H, J = 5.7, 2.7, 0.9);
13C NMR (125 MHz, CDCl
3) δ: 26.1, 26.5, 26.6, 28.1, 61.0, 70.6, 73.52, 73.53, 77.5, 104.7, 109.6, 112.0; HRMS (ESI) Calcd for C
12H
20O
6 [M+Na]
+: 283.1152; Found: 283.1149;
[α]D25−144.2 (CHCl3, c 1.0).
7.
Mallinckrodt Grade 564 CCGT3A molecular sieves are used without further activation.
8.
The mixture turns from orange-brown to a dark brown color during the course of the reaction, indicating the reduction of Cr(VI) to Cr(III).
9.
Slow addition of
ether is necessary for a high yield. The addition of
ether leads to the precipitation of only a small amount of the reduced
chromium. This filtration mainly removes the molecular sieves and
chromium species adsorbed during stirring.
10.
The yield of the ketone will be reduced if all the brown solids are not loaded onto the column (these solids contain some of the ketone).
11.
The product exhibits the following physical and spectral properties:
mp 101-103°C; IR (KBr) cm
−1: 1749;
1H NMR (500 MHz, CDCl
3) δ: 1.40 (s, 6 H), 1.46 (s, 3 H), 1.55 (s, 3 H), 4.00 (d, 1 H, J = 9.5), 4.12 (d, 1 H, J = 13.4), 4.39 (dd, 1 H, J = 13.4, 2.2), 4.55 (ddd, 1 H, J = 5.7, 2.2, 1.0), 4.61 (d, 1 H, J = 9.5), 4.73 (d, 1 H, J = 5.7);
13C NMR (125 MHz, CDCl
3) δ: 26.20, 26.24, 26.7, 27.3, 60.3, 70.2, 76.1, 78.1, 104.3, 110.8, 114.0, 197.1; HRMS (ESI) Calcd for C
12H
18O
6[M+Na]
+: 281.0996; Found: 281.0985;
[α]D25−125.4 (CHCl3, c 1.0).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Dioxiranes are remarkably versatile oxidizing agents which show encouraging potential for asymmetric synthesis, particularly asymmetric epoxidation. Dioxiranes can be generated in situ from Oxone (KHSO
5) and ketones (Scheme 1).
3 In principle, only a catalytic amount of ketone is required, so with a chiral ketone there exists the opportunity for catalytic asymmetric epoxidation.
4,5 Since the first asymmetric epoxidation of olefins with a chiral dioxirane reported by Curci in 1984,
4a this area has received intensive interest and significant progress has been made.
4,5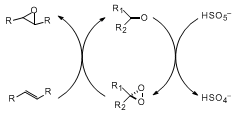
The fructose-derived ketone (2) described herein is a member of a class of ketones designed to contain the following general features: (1) The stereogenic centers are close to the reacting center, resulting in efficient transfer of stereochemistry between substrate and catalyst. (2) The presence of fused ring(s) or a quaternary center α to the carbonyl group minimizes epimerization of the stereogenic centers. (3) Approach of an olefin to the reacting dioxirane can be controlled by sterically blocking one face or using a C2- or pseudo-C2-symmetric element. (4) Electron-withdrawing (by induction) substitutents are introduced to activate the carbonyl.
Ketone
2 gives very high enantioselectivities for a variety of trans-disubstituted and trisubstituted olefins.
5 The ketone catalyst can be readily synthesized from very inexpensive
D-fructose by ketalization with
acetone2e,h and subsequent oxidation of the remaining alcohol to the ketone. Other acids such as H
2SO
4 can also be used for ketalization.
2c,f,i Although the present procedure uses PCC for the oxidation, many other oxidants such as PDC-Ac
2O,
2f DMSO-Ac
2O,
2a,b,d DMSO-DCC,
2e DMSO-(COCl
2),
2g RuCl
3-NaIO
4,
2h Ru-TBHP,
2j etc. are also effective. The enantiomer of catalyst
2 (
ent-2) can be prepared in the same fashion from
L-fructose, which in turn can be prepared from readily available
L-sorbose.
6,5c As expected, the enantiomeric catalyst shows the same enantioselectivity in epoxidation reactions.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1,2:4,5-Di-O-isopropylidene-D-erythro-2,3-hexodiulo-2,6-pyranose:
β-D-erythro-2,3-Hexodiulo-2,6-pyranose, 1,2:4,5-bis-O-(1-methylethylidene)- (9); (18422-53-2)
D-Fructose (9); (57-48-7)
2,2-Dimethoxypropane:
Propane, 2,2-dimethoxy- (9); (77-76-9)
Perchloric acid (8, 9); (7601-90-3)
1,2:4,5-Di-O-isopropylidene-β-D-fructopyranose:
β-D-Fructopyranose, 1,2;4,5-bis-O-(1-methylethylidene)- (9); (25018-67-1)
Pyridinium chlorochromate:
Chromate(1-), chlorotrioxo-, (T-4)-, hydrogen compd. with pyridine (1:1) (9); (26299-14-9)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved