Org. Synth. 2003, 80, 111
DOI: 10.15227/orgsyn.080.0111
INTRA- AND INTERMOLECULAR KULINKOVICH CYCLOPROPANATION REACTIONS OF CARBOXYLIC ESTERS WITH OLEFINS: BICYCLO[3.1.0]HEXAN-1-OL AND trans-2-BENZYL-1-METHYLCYCLOPROPAN-1-OL
[(Bicyclo[3.1.0]hexan-1-ol and 1-Cyclopropanol, 1-methyl-2-phenylmethyl-)]
Submitted by Se-Ho Kim, Moo Je Sung, and Jin Kun Cha
1.
Checked by Fanglong Yang and Dennis P. Curran.
1. Procedure
A.
Bicyclo[3.1.0]hexan-1-ol (
1). To a
500-mL, round-bottomed flask, equipped with a
magnetic stirring bar and
rubber septum (Note 1), is added at room temperature a mixture of
2.0 g (15.6 mmol) of methyl 5-hexenoate,
11.2 mL (11.2 mmol) of a 1M solution of chlorotitanium triisopropoxide in
hexane, and 54 mL of anhydrous ether (Note 2) under a
nitrogen atmosphere. A 1M solution of
n-butylmagnesium chloride in ether (52 mL, 52 mmol; (Note 3)) is added over a period of 6.5 hr via a
syringe pump (Note 4) at room temperature. After the addition is complete, the resulting black reaction mixture is stirred for an additional 20 min. The mixture is cooled to 0°C with an
ice bath, diluted with
50 mL of ether and then quenched by slow addition of water (14 mL). The resulting mixture is stirred for an additional 3 hr at room temperature. The organic phase is separated and the aqueous phase is extracted with
ether (3 × 100 mL). The combined organic extracts are washed with
brine (2 × 50 mL), dried over
anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure using a
rotary evaporator (Note 5). Purification of the crude product
(Note 6) by column chromatography on 40 g of silica gel
(Note 7) using a gradient of
5% to 10% ether/pentane as the eluent provides
1.09 g (
71%) of
bicyclo[3.1.0]hexan-1-ol (
1)
2 as a colorless oil
(Note 8).
B. trans-2-Benzyl-1-methylcyclopropan-1-ol (2). To a 500-mL, round-bottomed flask, equipped with a magnetic stirring bar and rubber septum, is added a mixture of 2.5 g (21 mmol) of allylbenzene, 2 mL (20 mmol) of ethyl acetate, and 20 mL of a 1M solution of chlorotitanium triisopropoxide in hexane (Note 2), and 160 mL of anhydrous tetrahydrofuran (THF). After the mixture has been cooled to 0°C with an ice bath under a nitrogen atmosphere, a 1M solution of cyclopentylmagnesium chloride in ether (80 mL, 80 mmol; (Note 9)) is added over a period of 2.5 hr via a syringe pump (Note 4). After the addition is complete, the resulting black reaction mixture is stirred for 30 min at 0°C, then is quenched by the cautious addition of water (15 mL). The resulting mixture is stirred for an additional 1 hr at room temperature and filtered through a pad of Celite, which is rinsed thoroughly with ether (4 × 50 mL). The combined filtrate and rinsings are poured into a separatory funnel containing 50 mL of water and shaken thoroughly. The organic phase is separated, washed with brine (50 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure using a rotary evaporator. Purification of the crude product (obtained as a pale yellow oil) by column chromatography on 80 g of silica gel (Note 7) using 1:20 ethyl acetate:hexane as the eluent provides 2.6 g (80%) of trans-2-benzyl-1-methylcyclopropan-1-ol (2) as a colorless oil (Note 10).
2. Notes
1.
All glassware, needles, syringes were oven-dried (>100°C) and quickly assembled prior to use.
2.
(a)
Methyl 5-hexenoate was purchased from TCI America and used as received. A 1M solution of
chlorotitanium triisopropoxide in hexane was purchased from Aldrich Chemical Company, Inc. and used as obtained. (b)
Ether and THF were dried and purified by distillation from
sodium/
benzophenone. Unless stated otherwise, all solvents and reagents in this procedure were obtained commercially and used as received.
3.
n-Butylmagnesium chloride (a 2M solution in ether) was available from Aldrich Chemical Company, Inc. Alternatively, it was freshly prepared from
1-chlorobutane and
magnesium.
4.
(a) The tip of a syringe needle was placed right above the surface of the reaction mixture, rather than near the neck of the reaction flask. This placement was found to minimize the amount of the Grignard reagent to be added. An excess of the Grignard reagent was used to ensure complete conversion. (b) To prevent the glass syringe plunger from sticking in the barrel, vacuum grease was applied around the edge of the exposed portion of the plunger.
5.
The solvent was removed under water (aspirator) vacuum, while the bath temperature was kept below 20°C. Otherwise, considerable loss occurred due to the volatility of the cyclopropanol product.
6.
Immediate purification of the crude product is recommended. Otherwise, it should be stored in a cold (−30°C) freezer to avoid decomposition.
7.
Flash chromatography was performed on E. Merck silica gel (70-230 mesh). For Procedure A, the checkers used 60 g silica gel, eluting first with
150 mL 10% ether/pentane, then with
200 mL 20% ether/pentane. For Procedure B, the checkers used 100 g silica gel and eluted first with
100 mL 9% ether/hexane, then with
200 mL 17% ether/hexane.
8.
Yields in several runs were obtained in the range of
68-75% by the submitters and
67-71% by the checkers. Spectral data for
1 [R
f = 0.27 (1:4
ethyl acetate:hexane)] are as follows:
bp 53-55°C (15 mm, Kugelrohr) [lit.
2b bp 63-65°C (20 mm)]; IR (neat) cm
−1: 3300;
1H NMR (360 MHz, acetone-d
6) δ 0.47 (t, J = 4.6, 1 H), 0.67 (dd, J = 4.6, 8.9, 1 H), 1.02-1.15 (m, 2 H), 1.43 (dd, J = 7.9, 12.1, 1 H), 1.55 (m, 1 H), 1.75-1.89 (m, 3 H), 4.25 (s, 1 H, -OH);
13C NMR (90 MHz, acetone-d
6) δ 14.9, 21.8, 24.3, 27.4, 34.3, 64.2; Mass spectrum: m/z 98 (M
+, 29), 97 (47), 83 (56), 70 (100), 55 (54).
9.
Cyclopentylmagnesium chloride (a 2M solution in ether) was available from Aldrich Chemical Company, Inc. Alternatively, it was freshly prepared from
chlorocyclopentane and
magnesium.
10.
Yields in several runs were obtained in the range of
78-86% by the submitters and
78-82% by the checkers. Spectral data for
2 [R
f = 0.47 (1:3
ethyl acetate:
hexane)] are as follows: IR (neat) cm
−1: 3332, 1228;
1H NMR (360 MHz, CDCl
3) δ 0.29 (dd, J = 5.4, 6.0, 1 H), 0.98 (dd, J = 5.4, 10.0, 1 H), 1.30-1.39 (m, 1 H), 1.49 (s, 3 H), 1.88 (s, 1 H, -OH), 2.55 (dd, J = 7.5, 15.3, 1 H), 2.67 (dd, J = 7.3, 15.3, 1 H), 7.19-7.33 (m, 5 H);
13C NMR (90 MHz, CDCl
3) δ 20.4, 20.8, 26.1, 35.6, 55.8, 125.9, 128.1, 128.4, 141.6; Mass spectrum (EI): m/z 162 (M
+, 3), 104 (76), 92 (58), 71 (100).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Kulinkovich and co-workers recently developed a new method for preparing cyclopropanols (the subject of recent reviews
3) by treating carboxylic esters with a suitable Grignard reagent (i.e., containing β-
hydrogen atoms) in the presence of
titanium tetraisopropoxide.
4 The present procedures represent a convenient variant of the Kulinkovich cyclopropanation reaction by using a sacrificial Grignard reagent (
n-butylmagnesium chloride or
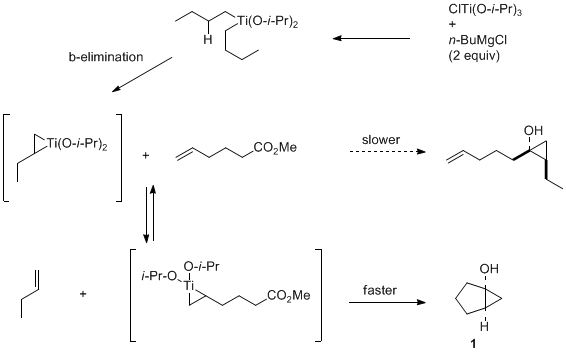
cyclopentylmagnesium chloride) to generate the putative titanacyclopropane intermediate for subsequent ligand exchange with a monosubstituted olefin. A mixture of the two rapidly interconverting titanacyclopropane intermediates is thus formed, followed by the intramolecular cyclopropanation reaction, to afford the corresponding bicyclic cyclopropanol products in good to excellent yield.
5,6 Because formation of five- and six-membered rings proceeds much faster than the intermolecular reaction, the precise position of equilibrium is inconsequential for successful cyclization, as demonstrated by the facile preparation of
bicyclo[3.1.0]hexan-1-ol (
1). Consequently, various Grignard reagents have been shown to be effective for the intramolecular cyclopropanation reactions with insignificant differences in yields. Whereas several titanium alkoxides and aryloxides can also be employed,
chlorotitanium triisopropoxide and
methyltitanium triisopropoxide have often been found to be the
titanium reagent of choice.
7 Ether, THF,
toluene, or even
dichloromethane are generally appropriate reaction solvents.
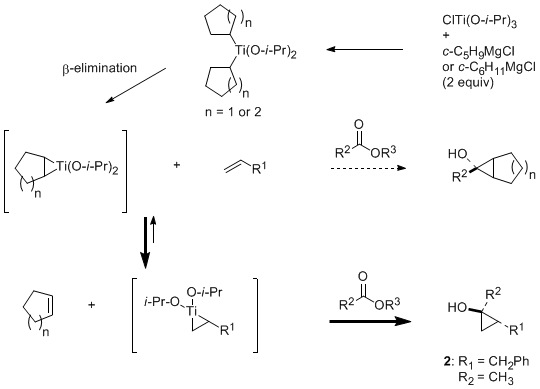
Central to the successful extension of the olefin exchange strategy to intermolecular coupling reactions of an ester and a monosubstituted olefin, as illustrated by the preparation of
trans-2-benzyl-1-methylcyclopropan-1-ol (
2), is the use of cyclohexyl or cyclopentyl Grignard reagents.
8 These secondary Grignard reagents are believed to allow the position of equilibrium to be shifted to the desired titanacyclopropane intermediate (less substituted, more stable). Notable characteristics of the ligand exchange-mediated variant of the Kulinkovich cyclopropanation include the simplicity and ease of operation using inexpensive reagents and also excellent compatibility with several common functional groups (for example, alkoxy, bromo, and alkenyl substituents).
In addition, other carboxylic acid derivatives, such as tertiary carboxamides and cyclic carbonates, readily undergo cyclopropanation reactions under similar conditions to provide cyclopropylamines
9,10 and cyclopropanone hemiacetals,
11 respectively. The cognate titanium-mediated coupling of imides has been shown to afford synthetically useful N-acylhemiaminals.
12
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Bicyclo[3.1.0]hexan-1-ol (8, 9); (7422-09-5)
Methyl 5-hexenoate:
5-Hexenoic acid, methyl ester (8, 9); (2396-80-7)
Chlorotitanium triisopropoxide:
Titanium, chlorotris(2-propanolato)-, (T-4)- (9); (20717-86-6)
n-Butylmagnesium chloride:
Magnesium, butylchloro- (8, 9); (693-04-9)
Allylbenzene:
Benzene, 2-propenyl- (9); (300-57-2)
Cyclopentylmagnesium chloride:
Magnesium, chlorocyclopentyl- (8, 9); (32916-51-1)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved