Org. Synth. 2005, 81, 26
DOI: 10.15227/orgsyn.081.0026
dl-SELECTIVE PINACOL-TYPE COUPLING USING ZINC, CHLOROSILANE, AND CATALYTIC AMOUNTS OF Cp2VCl2; dl-1,2-DICYCLOHEXYLETHANEDIOL
[(1,2-Ethanediol,1,2-dicyclohexyl-)]
Submitted by Toshikazu Hirao, Akiya Ogawa, Motoki Asahara, Yasuaki Muguruma, and Hidehiro Sakurai
1.
Checked by Helga Krause and Alois Fürstner.
1. Procedure
A 500-mL, two-necked, round-bottomed flask equipped with a rubber septum fitted with an argon inlet needle, a 200-mL, pressure-equalizing addition funnel capped with a rubber septum, and a magnetic stirbar is charged with zinc powder (13 g, 200 mmol) (Note 1) and dichlorodicyclopentadienylvanadium (0.756 g, 3.0 mmol) (Note 2). The flask is flame-dried and purged with argon, allowed to cool to room temperature, and then a solution of chlorotrimethylsilane (21.7 g, 200 mmol) (Note 3) in 200 mL of tetrahydrofuran (THF) (Note 4) is added dropwise over ca. 15 min via the addition funnel. The reaction mixture is stirred at room temperature for 1 hr during which time the color of the solution changes from red purple to light blue. Cyclohexanecarboxaldehyde (11.2 g, 100 mmol) (Note 5) is added via syringe over 5 min and the reaction mixture is stirred at room temperature for 13 hr. Diethyl ether (100 mL) and 100 mL of 2M aqueous HCl solution are added to the resulting mixture. The organic phase is separated and washed with 50 mL of saturated aqueous sodium bicarbonate solution, two 50-mL portions of water, and 50 mL of brine, dried over sodium sulfate, and concentrated by rotary evaporation to give 9.86-10.79 g (87-95%) of 1,2-dicyclohexylethanediol as a mixture of diastereomers (dl/meso = 87/13, (Note 6)). Three to four consecutive recrystallizations from ethanol (10 mL/g) affords 2.3-3.4 g (20-30%) of the dl-isomer in pure form (Notes 7,8).
2. Notes
1.
Zinc powder was purchased from Wako Pure Chemical Industries and used as received.
2.
Dichlorodicyclopentadienylvanadium was purchased from Aldrich Chemical Company, Inc. and used as received.
3.
Chlorotrimethylsilane was purchased from Wako Pure Chemical Industries and freshly distilled from
CaH2 before use.
4.
THF was obtained from Kanto Chemical Co., Inc. as dehydrated stabilizer-free grade.
5.
Cyclohexanecarboxaldehyde was purchased from Tokyo Chemical Industry Co., Ltd. and used as received.
6.
The diastereomeric ratio was determined by the NMR integral ratio of the methine protons adjacent to the hydroxyl group (3.33 ppm for the
dl-isomer; 3.44 ppm for the
meso-isomer).
7.
The checkers obtained
6.7 g (
60%) after the first recrystallization; this sample showed a
dl/meso ratio of 95:5. The submitters report obtaining
5.5-7.0 g (
49-62%) of the pure
dl isomer after three recrystallizations.
8.
The physical properties of the
dl-isomer are as follows:
mp 136-138°C; IR (KBr) 3320, 2915, 2850, 1450, 1410, 1270, 1105, 1020, 890, 735 cm
−1;
1HNMR (400 MHz, CDCl
3) δ 1.0-1.3 (m, 10H), 1.5-1.9 (m, 14H), 3.33 (br, 2H);
13CNMR (100 MHz, CDCl
3) δ 26.1, 26.3, 26.5, 28.3, 29.7, 40.5, 75.2; MS (EI)
m/z (rel. intensity): 226 (0.7, [M
+]), 143 (17), 113 (50), 112 (40), 96 (13), 95 (100), 81 (10), 67 (10), 55 (16), 41 (11); Anal. Found: C, 74.18; H, 11.52%. Calcd for C
14H
26O
2 : C, 74.29; H, 11.58%.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Metal-induced reductive dimerization of carbonyl compounds is a useful synthetic method for the formation of vicinally functionalized carbon-carbon bonds. For stoichiometric reductive dimerizations, low-valent metals such as
aluminum amalgam, titanium, vanadium, zinc, and samarium have been employed. Alternatively, ternary systems consisting of
catalytic amounts of a metal salt or metal complex, a chlorosilane, and a stoichiometric co-reductant provide a catalytic method for the formation of pinacols based on reversible redox couples.
2 The homocoupling of aldehydes is effected by vanadium or titanium catalysts in the presence of Me
3SiCl and Zn or Al to give the 1,2-diol derivatives; high selectivity for the
dl-isomer is observed in the case of secondary aliphatic or aromatic aldehydes.
A variety of such ternary catalytic systems has been developed for diastereoselective carbon-carbon bond formations (Table). A Cp-substituted vanadium catalyst is superior to the unsubstituted one,
3 whereas a reduced species generated from VOCl
3 and a co-reductant is an excellent catalyst for the reductive coupling of aromatic aldehydes.
4 A trinuclear complex derived from
Cp2TiCl2 and
MgBr2 is similarly effective for
dl-selective pinacol coupling.
5 The observed
dl-selectivity may be explained by minimization of steric effects through
anti-orientation of the bulky substituents in the intermediate.
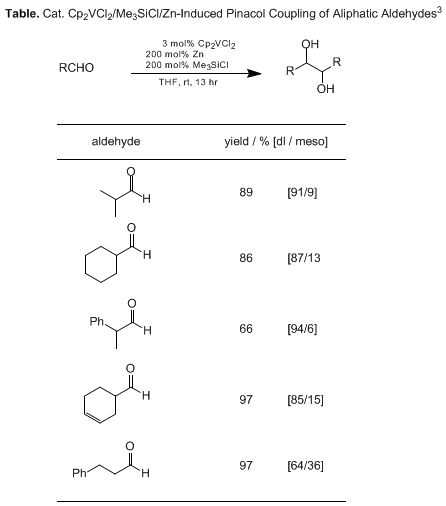
Chlorosilanes appear to contribute to the catalytic reactions in various ways. Importantly, they are necessary to liberate the catalyst from the primary product formed and they are also thought to facilitate the electron transfer to the carbonyl moiety, generating the stabilized silyloxyalkyl radicals that subsequently undergo dimerization. Moreover, the observed diastereoselectivity partly depends on the substituents of the chlorosilanes. Similar ligand and additive effects are observed in diastereoselective titanium-catalyzed coupling reactions of aromatic aldehydes.
6,7 Use of water as a solvent does not require the chlorosilanes as an additive.
8The cat.
Cp2VCl2/R
3SiCl/
Zn system outlined above can also be used for the reductive coupling of aldimines with
meso-diastereoselectivity.
9 The observed selectivity depends on the substituents on the nitrogen as well as the silicon atoms. 1,5- and 1,6-dialdehydes undergo intramolecular pinacol coupling to give cyclic
vic-diols with excellent selectivity.
3,6 The reductive coupling has also been applied to the catalytic diastereoselective cyclization of arylidene malononitriles and ketonitriles to give the corresponding cyclopentene and cyclopentenol derivatives, respectively.
10
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Dichlorodicyclopentadienylvanadium;
Vanadium, dichlorobis (η5-2,4-cyclopentadien-1-yl)-; (12083-48-6)
Chlorotrimethylsilane:
Silane, chlorotrimethyl-; (75-77-4)
Cyclohexanecarboxaldehyde; (2043-61-0)
Zinc; (7440-66-6)
dl-1,2-Cyclohexylethanediol:
1,2-Ethanediol, 1,2-dicyclohexyl-; (92319-61-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved