Org. Synth. 2005, 81, 188
DOI: 10.15227/orgsyn.081.0188
INDIUM/AMMONIUM CHLORIDE-MEDIATED SELECTIVE REDUCTION OF AROMATIC NITRO COMPOUNDS: ETHYL 4-AMINOBENZOATE
[(Benzoic acid, 4-amino-, ethyl ester)]
Submitted by Bimal K. Banik
1, Indrani Banik, and Frederick F. Becker.
Checked by Weiqiang Huang and Marvin J. Miller.
1. Procedure
Ethyl 4-aminobenzoate. A 1000-mL, round-bottomed flask equipped with a magnetic stirbar is charged with a suspension of 10 g (51 mmol) of ethyl 4-nitrobenzoate in 250 mL of ethanol, and a solution of 27.4 g (510 mmol) of ammonium chloride in 125 mL of water is then added (Note 1). Indium powder (23.5 g, 205 mmol) (Note 2) is added, and the resulting mixture is heated at reflux for 2.5 hr. The reaction mixture is allowed to cool to room temperature, diluted with 350-400 mL of water, and filtered under vacuum. The filtrate is extracted with 6-8 portions of 50-60 mL of dichloromethane, and the combined organic phases are washed with 100 mL of brine and dried over anhydrous sodium sulfate. The resulting solution is concentrated under reduced pressure and the crude product is dissolved in 100 mL of dichloromethane. The solution is concentrated by warming, and 50 mL of hexane is then added. The resulting solution is allowed to stand in a refrigerator overnight and then filtered under vacuum to give 7.63 g (90%) of ethyl 4-aminobenzoate (Note 3).
2. Notes
1.
Ethyl 4-nitrobenzoate,
ammonium chloride,
ethanol were purchased from Aldrich Chemical Company and used as received.
2.
Indium powder (99.99%) was obtained from Aldrich Chemical Company.
3.
The spectral properties of
ethyl 4-aminobenzoate are as follows: IR (film) cm
−1: 3424, 3345, 3224, 1685, 1636, 1598, 1515, 1367, 1312, 1281, 1173, 773;
1H NMR
pdf (300 MHz, CDCl
3) δ: 1.36 (3 H, t, J = 6), 4.04 (2 H, brs), 4.31 (2 H, q, J = 7), 6.63 (2 H, d, J = 9), 7.85 (2 H, d, J = 9);
13C NMR (75 MHz, CDCl
3) δ: 14.6 (CH
3), 60.4 (CH
2), 114.0 (Ar-CH), 120.5 (Ar-q), 131.7 (Ar-CH), 151.0 (Ar-q), 166.9 (Ar-q = quaternary carbon atom);
m/e: 166 (M+H)
+; exact mass: m/e (M
+): 165.0764 (measured), 165.0790 (theoretical).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The synthesis of aromatic amines is an active and important area of research.
2 Many methods are available in the literature for the synthesis of these compounds. Though some of these are widely used, still they have limitations based on safety or handling considerations. For example, catalytic hydrogenation
3 of nitro or azido compounds in the presence of metals such as palladium on carbon or Raney nickel require stringent precautions because of their flammable nature in the presence of air. In addition, these methods require compressed hydrogen gas and a
vacuum pump to create high pressure within the reaction flask. To overcome these difficulties, several new methods have been reported in the recent literature
4 involving such reducing agents as
decaborane,
5 electrochemically generated Raney nickel,
6 dimethyl hydrazine/
ferric chloride,
7 hydrazine hydrate/
ferric oxide-
magnesium oxide,
8 diethyl chlorophosphite,
9 and
sodium borohydride-
sodium-methoxide in
methanol10 In general, the main drawbacks of these methods are long reaction time and non-chemoselectivity. The submitters
11 have also described new methods for the reduction of aromatic nitro compounds and imines to aromatic amines by novel
samarium-induced iodine catalyzed and
ammonium chloride mediated reduction.
12 While our
samarium-induced reduction of aromatic nitro compounds works well in the polycarbocyclic series, similar reaction with several heteroaromatic nitro compounds results in a mixture of products under identical conditions. Moreover,
samarium-induced reduction of the nitro compounds requires anhydrous reaction conditions.
The submitters have been actively involved in the use of polyaromatic amines for the development of anticancer agents.
13 Therefore, the submitters began a research program aimed at developing methods to synthesize several aromatic amines rapidly and in high selectivity, by using ecologically friendly reagents. The present study describes a method for the selective reduction of aromatic nitro compounds to the corresponding amines by
indium in the presence of
ammonium chloride in aqueous
ethanol.
14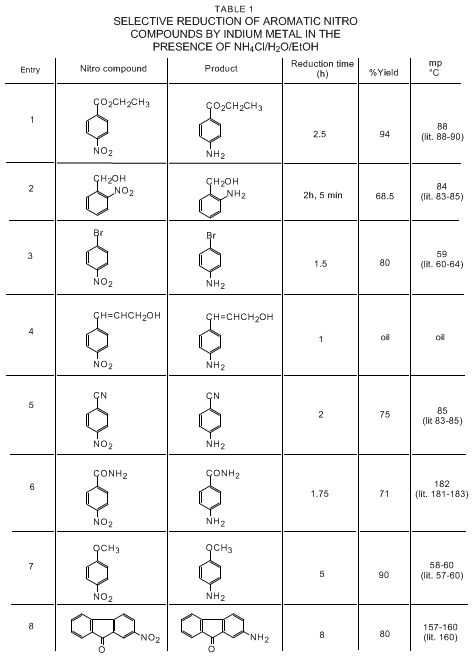
The chemistry of
indium metal is the subject of current investigation, especially since the reactions induced by it can be performed in aqueous solution.
15 The selective reductions of
ethyl 4-nitrobenzoate (entry 1),
2-nitrobenzyl alcohol (entry 2),
1-bromo-4-nitrobenzene (entry 3),
4-nitrocinnamyl alcohol (entry 4),
4-nitrobenzonitrile (entry 5),
4-nitrobenzamide (entry 6),
4-nitroanisole (entry 7), and
2-nitrofluorenone (entry 8) with
indium metal in the presence of
ammonium chloride using aqueous
ethanol were performed and the corresponding amines were produced in good yield. These results indicate a useful selectivity in the reduction procedure. For example, ester, nitrile, bromo, amide, benzylic ketone, benzylic alcohol, aromatic ether, and unsaturated bonds remained unaffected during this transformation. Many of the previous methods produce a mixture of compounds. Other metals like zinc, tin, and iron usually require acid-catalysts for the activation process, with resultant problems of waste disposal.
Because of the non-flammable nature of the process and ready availability of indium and ammonium chloride, the submitters believe this method is practical for the preparation of several aromatic amines. Further, this method is performed in aqueous ethanol, is extremely safe from the environmental point of view, and should prove useful in organic chemistry.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Ethyl 4-aminobenzoate:
Benzoic acid, 4-amino-, ethyl ester; (94-09-7)
Ethyl 4-nitrobenzoate:
Benzoic acid, 4-nitro-, ethyl ester; (99-77-4)
Ammonium chloride (NH4Cl); (12125-02-9)
Indium; (7440-74-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved