Org. Synth. 2006, 83, 18
DOI: 10.15227/orgsyn.083.0018
OXIDATION OF NEROL TO NERAL WITH IODOBENZENE DIACETATE AND TEMPO
[(Z)-3,7-Dimethyl-2,6-octadienal]
Submitted by Giovanni Piancatelli and Francesca Leonelli
1a.
Checked by Nga Do and John Ragan
1b.
1. Procedure
(Z)-3,7-Dimethyl-2,6-octadienal. A 250-mL round-bottomed flask equipped with a Teflon-coated magnetic stirring bar is charged with the following order of the reagents: acetonitrile (28 mL) (Note 1), (Z)-3,7-dimethyl-2,6-octadien-1-ol (nerol) (5.70 mL, 32.5 mmol) (Note 2), aqueous pH 7.0 buffer solution (8 mL) (Note 3), 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) (490 mg, 3.24 mmol) (Note 4), and iodobenzene diacetate (IBD) (11.49 g, 35.71 mmol) (Note 5). The reaction mixture is stirred at 0 °C (Note 6) until nerol is no longer detectable by TLC analysis (Note 7). The reaction mixture is diluted with diethyl ether (100 mL) and transferred to a 500-mL separatory funnel. The orange mixture is washed with saturated aqueous sodium thiosulfate (2 × 50 mL) (Note 8). The aqueous phase is separated and extracted with diethyl ether (3 × 35 mL). The combined organic layers are washed with saturated aqueous sodium hydrogen carbonate (40 mL) and then with saturated aqueous sodium chloride (40 mL) (Note 9). The organic layer is dried over anhydrous sodium sulfate, filtered, and concentrated with a rotary evaporator (35 °C, 70 mmHg). The residue is purified by column chromatography on silica gel (Note 10), using a 1:9 mixture of diethyl ether and hexanes as eluent (Note 11) to afford 4.30–4.39 g (87–89%) of (Z)-3,7-dimethyl-2,6-octadien-1-al (neral) as a colorless oil (Note 12). The material is homogenous by TLC, IR, 1H and 13C-NMR (Note 13).
2. Notes
1.
"RPE-For analysis"-grade CH3CN, as supplied by Carlo Erba Reagents (Italy), was used.
2.
Nerol (97%) was purchased from Aldrich and used directly without purification. The compound revealed traces of
geraniol (<3%) from GCMS analysis (Shimadzu GCMS-QP5000; EQUITY
TM-5 FUSED SILICA Capillary Column 30 m × 0.32 mm × 0.25 µm film thickness; 80 °C (1 min), 80-240 °C (16 min), 240 °C (1 min)); t
nerol = 8.43, t
rgeraniol = 8.75. The checkers used an Agilent HP-5 fused silica capillary column, 30 m × 0.32 mm × 0.25 µm; 250 °C detector, 100 °C isothermal program at 2 mL/min. This system gave the following retention times: t
nerol = 7.3 min, t
neral = 7.8 min, t
geraniol = 8.4 min, t
geranial = 9.1 min. The checkers' lot of
nerol (also purchased from Aldrich) showed <0.5% geraniol contamination.
3.
The
pH 7.0 buffer solution was purchased from Fluka.
4.
TEMPO (98%) was purchased from Aldrich and used as received.
5.
IBD (98%) was purchased from Aldrich and used as received.
6.
An ice-bath is placed under the reaction flask after the dissolution of
IBD (~ 3 min) because the reaction is slightly exothermic. On a smaller scale (1-5 mmol) this precaution is not necessary. The checkers placed the reaction flask in an ice bath prior to addition of the
TEMPO and
IBD; as the
IBD began dissolving, an internal temperature probe showed a temperature rise of 6–9 °C over the course of the reaction. This was not an issue on the scale run, but should be carefully monitored if the reaction were to be significantly increased in scale.
7.
The oxidation reaction is usually very fast and is complete after 20 min on the scale described above. The reaction was checked by TLC to confirm completion. Thin-layer chromatography analysis was carried out on E. Merck silica gel F254 plates by elution with
Et2O/hexanes (3/7), then sprayed with
2 N H2SO4 solution and heated with a hot plate for 1 min. The alcohol starting material has an R
f = 0.29 (light brown) and the aldehyde product has an R
f = 0.66 (deep purple). The reaction can also be monitored by GC/MS, as indicated by the checkers in
Note 3.
8.
Washing with saturated aqueous
sodium thiosulfate removes
TEMPO from the organic phase that becomes light yellow. If the organic phase should be still orange after the two washings, the solution is shaken in the
separatory funnel with
50 mL of saturated aqueous sodium thiosulfate and
15 mL of 0.5 N HCl until it becomes light yellow (the checkers found this extra thiosulfate-HCl wash to be necessary).
TEMPO must be removed carefully at this stage, because it cannot be removed in the chromatographic purification.
9.
The aqueous phase must be neutral. Acidic impurities can catalyze (
E)/(Z) isomerization of the aldehyde in the purification stage.
10.
Silica gel 60 (0.063-0.2 mm/ 70-230 mesh ASTM), purchased from Macherey-Nagel, was used. The column chromatography was performed using a 1/20 ratio between the crude product and silica gel.
11.
The solvent required for the purification of the product varies from 1.4 to 1.6 L. The chromatographic solvent is removed with a
rotary evaporator (35 °C, 70 mmHg).
12.
The checkers used
diethyl ether:
hexane (2:8) for column elution. Attempted purification by distillation (bp 118–120 °C, 20 mmHg) led to significant olefin isomerization (the pot residue was a 50:50 mixture of neral and
geranial).
13.
The following analytical data have been obtained for
(Z)-3,7-dimethyl-2,6-octadien-1-al: IR (CHCl
3) cm
−1: 1675 (C≡O), 1635 (C≡C);
1H NMR
pdf (400 MHz, CDCl
3) δ: 1.58 (s, 3H), 1.67 (s, 3H), 1.97 (d,
J = 1.2 Hz, 3H), 2.15–2.25 (m, 2 H), 2.57 (t,
J = 7.5 Hz, 2 H), 5.06–5.11 (m, 1 H), 5.86 (d,
J = 8.3 Hz, 1 H), 9.88 (d,
J = 8.3 Hz, 1 H);
13C NMR (100 MHz, CDCl
3) δ: 17.9, 25.3, 25.9, 27.2, 32.7, 122.4, 128.8, 133.9, 164.1, 191.0; GCMS purity >97%, t
r = 8.56. The compound revealed traces of geranial (<3%, t
r = 8.98) from GCMS analysis (See
Note 3).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The catalytic procedure described here illustrates a fast and inexpensive conversion of a (Z)-configured primary allylic alcohol into the corresponding (Z)-configured α,β-unsaturated aldehyde in high yields. The result demonstrates the chemoselectivity of the process, in that the easily isomerizable Z-configuration of the nerol is maintained.
Various methods for the oxidation of
nerol to
neral are available. Although individually having some synthetic advantages, most methods suffer from one or more experimental drawbacks, such as severe or delicate reaction conditions, complicated reaction procedures, and the need to use toxic or unstable reagents.
2Oxammonium salts
1 are the effective oxidant species derived from TEMPO, and have been used extensively either in stoichiometric or in catalytic amounts for the oxidation of primary and secondary alcohols to the corresponding carbonyl compounds.
3 Compound
1 has been generated
in situ from nitroxyl radicals, such as TEMPO, in combination with a number of secondary oxidants.
4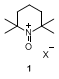
Hypervalent iodine reagents have been used recently for a variety of organic transformations.
4 Inter alia,
IBD in combination with catalytic amounts of
TEMPO is used as a stoichiometric oxidant in the conversion of primary and secondary alcohols to carbonyl compounds.
5 This oxidation protocol works efficiently at room temperature in
dichloromethane (and also in most common organic solvents and neat in some cases) and can be
Table 1. Oxidation of Primary Alcohols to Aldehydes5
|
|
|
performed in an open flask without special precautions (
e.g. inert atmosphere or dry solvent). This process exhibits a very high degree of selectivity for the oxidation of primary alcohols to aldehydes, without noticeable over-oxidation to carboxyl compounds, and a high chemoselectivity in the presence of either secondary alcohols or of other oxidizable moieties.
5Many sensitive protective groups are not affected by this method. Some examples of carbonyl compounds synthesized with this method are reported in Table 1.
5
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(Z)-3,7-Dimethyl-2,6-octadien-1-ol (
nerol); (106-25-2)
2,2,6,6-Tetramethylpiperidin-1-oxyl (TEMPO):
2,2,6,6-Tetramethyl-1-piperidinyloxy; (2564-83-2)
Iodobenzene diacetate (IBD):
Bis(acetato-κO)phenyliodine; (3240-34-4)
(Z)-3,7-Dimethyl-2,6-octadien-1-al (
neral):
(2Z)-3,7-Dimethyl-2,6-octadienal; (106-26-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved