Org. Synth. 2006, 83, 170
DOI: 10.15227/orgsyn.083.0170
ALLYLBORONATION OF IMINES: 1-PHENYLHEX-5-EN-3-AMINE
Submitted by Masaharu Sugiura, Keiichi Hirano, and Shu¯ Kobayashi
1.
Checked by Nai-Wen Tseng and Mark Lautens.
1. Procedure
Caution! The reaction should be conducted in a well-ventilated hood.
An oven-dried, 250-mL, two-necked, round-bottomed flask is charged with dodecylbenzenesulfonic acid (1.307 g, 4.0 mmol) (Note 1), flushed with argon, and equipped with a magnetic stirring bar, a rubber septum, and an argon inlet. The flask is charged with 80 mL of 28% aqueous ammonia (Note 2) by syringe. After the gas evolution ceases, the mixture is stirred at 23–25 °C to give a clear solution, then cooled to 0 °C in an ice-water bath. 2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2) (8.120 g, 9.0 mL, 48.3 mmol) (Note 3) is added dropwise over 5 min via a syringe (Note 4) and the mixture is stirred at 23–25 °C for 30 min. 3-Phenylpropionaldehyde (1) (5.328 g, 5.3 mL, 39.7 mmol) (Note 5) is added dropwise via a syringe over 5 min at 23–25 °C. The slurry mixture is vigorously stirred at 23–25 °C for 6 h (Note 6) and transferred to a 1-L separatory funnel with water (100 mL) and saturated aqueous NaCl solution (200 mL). The aqueous layer is extracted three times with diethyl ether (200, 150, and 150 mL), and the combined organic layers are washed with saturated aqueous NaCl solution (200 mL), dried over anhydrous sodium carbonate (Na2CO3), filtered, and concentrated under reduced pressure to afford crude amine 3 as a yellow oil (Note 8). The crude material is chromatographed on a 5.5 × 45 cm column containing 300 g of silica gel (Note 9). A mixture of hexane/isopropyl amine (20/1) is used as the eluent. Fractions (27 mL each) are collected in 30-mL test tubes (Note 10). Fractions containing the product are evaporated under reduced pressure (30 °C, 200 mmHg) to afford the amine as yellow oil. In order to completely remove the pinacol, the amine is diluted with diethyl ether (200 mL) and extracted three times with 1 M hydrochloric acid (3 × 50 mL). The combined aqueous layers are washed twice with diethyl ether (2 × 100 mL), carefully basified with 6 M sodium hydroxide (60 mL) (Note 7), and extracted three times with dichloromethane (200, 150, and 150 mL). The combined organic layers are dried over anhydrous sodium carbonate (Na2CO3), filtered, concentrated under reduced pressure (30 °C, 200 mmHg) and dried under vacuum (approximately 0.2 mmHg, 15 min) to afford 6.680 g of 3 (96%) as yellow oil (Note 11).
2. Notes
1.
Dodecylbenzenesulfonic acid (soft type) (>90.0%) was purchased from Tokyo Kasei Kogyo Co., Ltd. or TCI America, and used without purification. This viscous liquid material was measured directly in the reaction flask using a 5-mL pipette.
2.
Aqueous
ammonia solution (25.0-27.9%) was purchased from Wako Pure Chemical Industries, Ltd. and used as received. The checkers used aqueous
ammonia solution (28.0-30.0%) purchased from Aldrich Chemical Company, Inc.3.
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2) (95%) was purchased from Aldrich Chemical Company, Inc. This material included white precipitates. Therefore, it was filtered with
diethyl ether, evaporated (30 °C, 200 mmHg), and distilled (
72-73 °C, 27 mmHg, the boiling point is uncorrected) prior to use.
4.
On addition of
2, colorless precipitates were formed to give a slurry mixture.
5.
3-Phenylpropionaldehyde (1) (>90% GC) was purchased from Tokyo Kasei Kogyo Co., Ltd. and used after distillation. The checkers purchased this reagent from Aldrich Chemical Company, Inc.6.
As the reaction progressed, the precipitates gradually dissolved to give a cloudy solution. The reaction could be monitored by TLC analysis on Merck silica gel 60 F
254 plates and visualization with UV and
phosphomolybdic acid [
sodium phosphomolybdate hydrate (9.7 g) in
85% phosphoric acid (6.0 mL), concentrated
sulfuric acid (20 mL), and
water (400 mL)]. The checkers used 5%
phosphomolybidic acid in
ethanol as the TLC indicator. Disappearance of
1 was observed within 30 min. Compound
1 has R
f = 0.68 with hexane/ethyl acetate (3/1) as eluent (UV absorption, gray spot). Compound
3 has R
f = 0.13 with
hexane/
ethyl acetate (1/1) as eluent and R
f = 0.63 with
hexane/
isopropyl amine (10/1) as eluent (UV absorption, white spot). Pinacol has R
f = 0.30 with
hexane/
ethyl acetate (1/1) as eluent and R
f = 0.33 with
hexane/
isopropyl amine (10/1) as eluent (UV inactive, blue spot).
7.
After addition of
sodium hydroxide, the pH was checked by a pH-test paper to be approximately 9.
8.
The crude material was a mixture of
3 and pinacol (ca. 10/1 molar ratio). Distillation under reduced pressure (88 °C, 3 mmHg) gave almost pure
3, but could not separate
pinacol completely.
9.
Silica gel 60 70-230 mesh ASTM (Merck Ltd.) was used. Checkers used
Silica gel 60 40-63 mm (EMD Chemicals, Inc.).
10.
The column fractions were checked by TLC analysis on Merck silica gel 60 F
254 plates with
hexane/
isopropyl amine (20/1) as eluent and visualization with UV and
phosphomolybdic acid. R
f values are given in
(Note 6).
11.
The physical properties of
3 are as follows:
1H NMR
pdf (300 MHz, CDCl
3) δ: 1.26 (brs, 2 H), 1.61 (dddd,
J = 13.6, 10.1, 7.9, 5.7 Hz, 1 H), 1.76 (dddd,
J = 13.5, 10.3, 6.2, 5.4 Hz, 1 H), 2.03 (dddt,
J = 13.8, 7.9, 7.9, 0.9 Hz, 1 H), 2.27 (dddt,
J = 13.7, 6.3, 4.8, 1.4 Hz, 1 H), 2.64 (ddd,
J = 13.6, 10.1, 6.2 Hz, 1 H), 2.75 (ddd,
J = 13.7, 10.2, 5.7 Hz, 1 H), 2.82 (tt,
J = 7.9, 4.6 Hz, 1 H), 5.09 (dm,
J = 10.0 Hz, 1 H), 5.10 (dm,
J = 17.2 Hz, 1H), 5.79 (dddd,
J = 17.2, 10.0, 7.9, 6.4 Hz, 1 H), 7.15–7.21 (m, 3 H), 7.25–7.30 (m, 2 H);
13C NMR
pdf (75 MHz, CDCl
3) δ: 32.6, 39.4, 42.7, 50.1, 117.4, 125.7, 128.31, 128.33, 135.6, 142.2; HR-ESIMS calcd for C
12H
18N (M + H
+) 176.1439, found 176.1435; Anal. Calcd for C
12H
17N: C, 82.23; H, 9.78; N, 7.99. Found: C, 81.92; H, 9.77; N, 7.89.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Addition of allylmetals to C=N double bonds is a useful synthetic method for formation of homoallylic amine derivatives.
2 However, in order to obtain synthetically versatile homoallylic primary amines, removal of substituents on the nitrogen is often necessary. To address this issue, hydrolytically labile
N-silyl,
N-boryl, or
N-metalloimines have been utilized frequently as precursors to the primary amines; however, pre-formation of those imines are required. In contrast, the method in this procedure is based on in situ formation of
ammonia-derived imines and subsequent chemoselective allylation.
4,5 Thus, homoallylic primary amines are directly obtained from the three components without incorporation and cleavage of
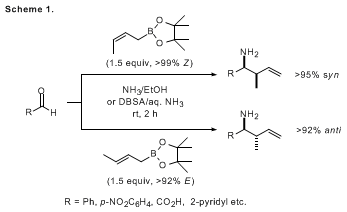
N-substituents. Additionally, when (
E)- and (
Z)-crotylboronates are used,
anti- and
syn-crotylated products can be obtained with high diastereoselectivities, respectively (Scheme 1).
Use of
ammonia as the
nitrogen source is key in this process. In contrast to
ammonia, primary amines scarcely undergo the reaction, and hence highly selective formation of homoallylic primary amines (suppression of over-reaction of the products) has been attained. Although
ammonia in
ethanol was utilized previously,
4 use of aqueous
ammonia in the presence of DBSA has made the reaction more practical.
5 A variety of additives were examined for the reaction of
3-phenylpropionaldehyde (1) with
allylboronate (2) in aqueous
ammonia. Among them, DBSA was found to be the best, whereas
lauric acid,
sodium dodecyl sulfate, and
sodium dodecylbenzenesulfonate were effective as well. In the absence of any additive, the yield of
3 and the chemoselectivity of the reaction (formation of
3 vs the corresponding homoallylic alcohol) were low. The hydrophobic alkyl chain of DBSA plays an important role, since
p-toluenesulfonic acid showed much lower activity. In reactions performed with DBSA, a variety of aliphatic, aromatic, heteroaromatic, and α,β-unsaturated aldehydes afford the corresponding homoallylic primary amines in good to excellent yields (Table 1).
5
|
|
entry
|
R (4)
|
time/h
|
%yield of 5a
|
|
|
1
|
Ph
|
6
|
61
|
|
2
|
p-NO2C6H4
|
6
|
60
|
|
3
|
p-MeOC6H4 |
6
|
60
|
|
4
|
o-HOC6H4
|
6
|
75
|
|
5
|
2-Pyridyl
|
6
|
88
|
|
6
|
3-Pyridyl
|
2
|
85
|
|
7
|
4-Pyridyl
|
2
|
83
|
|
8
|
2-Thienyl
|
6
|
60
|
|
9
|
3-Thienyl
|
2
|
95
|
|
10
|
2-Furyl
|
2
|
53
|
|
11
|
3-Furyl
|
2
|
93
|
|
12
|
(E)-PhCH=CH
|
2
|
78
|
|
13
|
c-C6H11
|
6
|
68
|
|
14
|
n-C5H11
|
2
|
49
|
|
|
|
However, addition of DBSA is not always necessary; hydrophilic carbonyl compounds such as α-oxo carboxylic acids and hydroxy aldehydes or ketones, including carbohydrates, were found to undergo the reactions smoothly with high selectivity as shown in Schemes 2 and 3.
6
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Dodecylbenzenesulfonic acid; (27176-87-0)
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane:
1,3,2-Dioxaborolane, 4,4,5,5-tetramethyl-2-(2-propenyl)-; (72824-04-5)
3-Phenylpropionaldehyde:
Benzenepropanal; (104-53-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved