Org. Synth. 2007, 84, 11
DOI: 10.15227/orgsyn.084.0011
GENERATION OF YNOLATE AND Z-SELECTIVE OLEFINATION OF ACYLSILANES: (Z)-2-METHYL-3-TRIMETHYLSILYL-2-BUTENOIC ACID
[(Z)-2-Butenoic acid, 2-methyl-3-trimethylsilyl-]
Submitted by Mitsuru Shindo
1, Kenji Matsumoto
2, and Kozo Shishido
2.
Checked by Scott E. Denmark and Brian M. Eklov.
1. Procedure
Caution! tert-Butylithium is extremely pyrophoric and must not be allowed to come into contact with the atmosphere. This reagent should only be handled by individuals trained in its proper and safe use. It is recommended that transfers be carried out by using a 20-mL or smaller glass syringe filled to no more than 2/3 capacity, or by cannula. For a discussion of procedures for handling air-sensitive reagents, see Aldrich Technical Bulletin AL-134. [Note added August 2009]
A.
Ethyl 2,2-dibromopropionate (
1).
3 A
1-L, three-necked, round-bottomed flask is equipped with a magnetic stirring bar and fitted with a thermocouple temperature probe
(Note 1) inserted through an adapter, a rubber septum, and a 100-mL, pressure-equalizing addition funnel fitted with a vacuum adapter which is capped with a rubber septum and the sidearm of which is connected to a vacuum/argon gas line. The flask is flame-dried under reduced pressure and then is maintained under an atmosphere of argon during the course of the reaction. The flask is charged with
diisopropylamine (23.0 mL, 164 mmol, 1.1 equiv) (Note 2) and
anhydrous THF (350 mL) (Note 3), and then is cooled to −71 °C (internal) in a dry ice/isopropyl alcohol bath
(Note 4) while a 1.62 M solution of
n-butyllithium in
hexane (102 mL, 165 mmol, 1.1 equiv) (Note 5) is added through the addition funnel over 10 min. After an additional 16 min, a solution of
ethyl 2-bromopropanoate (20.0 mL, 27.8 g, 154 mmol) (Note 6) in
anhydrous THF (20 mL) is added dropwise through the addition funnel over 18 min (the addition funnel is rinsed with
10 mL of anhydrous THF). After an additional 34 min, a solution of
1,2-dibromo-1,1,2,2-tetrachloroethane (53.7 g, 165 mmol, 1.1 equiv) (Note 7) in
THF (60 mL) is added rapidly in one portion through the addition funnel with vigorous stirring
(Note 8). After being stirred in the cold bath for 40 min
(Note 9), the mixture is poured into
150 mL of saturated aqueous sodium bicarbonate solution in a 1-L separatory funnel
(Note 10) and the mixture is extracted with
hexanes (2 × 150 mL). The combined organic layers are washed with
150 mL of water and
150 mL of brine, then are dried over
MgSO4 (6 g), filtered under vacuum, and concentrated by rotary evaporation (40 °C, 50–100 mmHg) to afford a dark brown oil. The oil is transferred to a
100-mL, round-bottomed flask equipped with a magnetic stirbar and fitted with a short-path distillation unit. Distillation of the product under reduced pressure using an oil bath affords 36.1 g (90% yield) of ethyl 2,2-dibromopropionate (
1) as a colorless liquid (71–80 °C / 17 mmHg) (Notes
11,
12) after an approximate 5 g forerun.
B.
(Z)-2-Methyl-3-trimethylsilyl-2-butenoic acid (
2).
4 A
500-mL, three-necked, round-bottomed flask is equipped with a magnetic stirring bar and fitted with a thermocouple temperature probe adapter, a rubber septum, and a pressure-equalizing, 200-mL addition funnel fitted with a vacuum adapter capped with a rubber septum. The side arm of the vacuum adapter is connected to a vacuum/argon gas system. The flask is flame-dried under reduced pressure and then maintained under an atmosphere of argon during the course of the reaction. The thermocouple is inserted through the adapter and the flask is charged with
ethyl 2,2-dibromopropionate (1, 17.6 g, 68.0 mmol, 1.2 equiv) and
anhydrous THF (180 mL) (Note 3). The solution is cooled with stirring to −72 °C (internal) in a dry ice/isopropyl alcohol bath, then a 1.72 M solution of
tert-butyllithium in
pentane (158 mL, 272 mmol, 4.9 equiv) (Note 13) is added dropwise over 67 min through the addition funnel such that the internal temperature is kept below −64 °C
(Note 14). The addition funnel is rinsed with
5 mL of THF. The yellow solution is stirred at −72 °C for an additional 110 min (3 h total reaction time), and then the cooling bath is replaced by an ice bath. After being stirred at 2 °C (internal) for 30 min, the ice bath is removed, and the mixture is allowed to warm to room temperature. A solution of
acetyltrimethylsilane (6.50 g, 56 mmol, Note 15) in
anhydrous THF (15 mL) is added through the addition funnel over 15 min
(Note 16). The addition funnel is rinsed with
5 mL of anhydrous THF, and the mixture is stirred for 45 min at room temperature. The reaction mixture is poured onto 1 M aqueous
NaOH (120 mL) in a 1-L separatory funnel. The organic phase is separated and the aqueous phase (pH 8) is washed with
ethyl acetate (2 × 120 mL). The combined organic extracts are washed with 1 M
aqueous sodium hydroxide solution (120 mL) and set aside. The combined aqueous extracts (pH 9) are acidified with 6 M
HCl (100 mL) to pH 1. The resulting cloudy suspension is extracted with
ethyl acetate (3 × 120 mL). These organic extracts are combined, washed with
brine (120 mL), dried over
MgSO4 (4 g), filtered through a coarse glass frit and then are concentrated on a rotary evaporator (23 °C, 10 mmHg) to yield 7.64 g of a viscous yellow-orange oil that solidifies upon standing in the freezer (−20 °C)
(Note 17). The crude product is transferred to a sublimation chamber (chamber: inside diameter, 57 mm; height, 90 mm; condenser: outside diameter, 27 mm; height 70 mm). The bottom of the chamber is cooled in an ice bath and the apparatus is attached to a vacuum line. The bath is removed once the majority of the volatile materials are evaporated as judged by stabilization of the pressure. When the pressure inside the chamber drops below 0.1 mmHg, the condenser is charged with a dry ice/isopropyl alcohol mixture and the chamber is placed in an oil bath, pre-heated to 40 °C. The sublimate was removed from the cold finger three times to afford 6.12 - 6.60 g (64–69%) of
2 as a white crystalline solid (mp 48.5- 50.5 °C,
Note 19).
2. Notes
1.
A PFA-coated thermocouple probe, Type K (Omega Engineering, Inc.) was inserted through the septum.
2.
Diisopropylamine was purchased from Sigma-Aldrich Company (>98.0%) and was distilled from calcium hydride.
3.
Anhydrous THF (>99.5%, stabilizer free) was purchased from Fischer Scientific and was used as received (Karl Fischer analysis gave < 120 μg/mL).
4.
For a small scale (smaller than
500-mL flask), the submitters used a low temperature bath with magnetic stirrer (Tokyo Rikakikai Co., Ltd., PSL-1800).
5.
n-Butyllithium solution was purchased from Sigma-Aldrich and was titrated by both No-D NMR spectroscopy
5a and Gilman double titration.
5b6.
Ethyl 2-bromopropanoate (>98%) was purchased from Tokyo Kasei Kogyo Co. Ltd. (TCI) and was distilled.
7.
1,2-Dibromo-1,1,2,2-tetrachloroethane (98%) was purchased from Alfa-Aesar and was used as received. 1,2-Dibromo-1,1,2,2-tetrafluoroethane (>99.0%, Tokyo Kasei Kogyo Co. Ltd.) is more convenient as a bromination reagent, but is not available in the USA.
8.
Addition required approximately 30 sec during which time the temperature rose from −71 °C to −35 °C. If this reagent was added slowly, a significant amount of side products were generated, probably due to self-condensation.
9.
The progress of the reaction was monitored by gas chromatography. GC-MS analysis was performed on a JMS-Automass SUN200 instrument equipped with a TC-17 30-m × 0.32-mm × 0.25-μm column under the following conditions: injector temp 250 °C; oven temp 60 °C, 3 min; ramp 8 °C/min; final temp 250 °C; helium gas flow 0.8 mL/min; t
R = 4.56 min (dibromo ester), t
R = 2.13 min (bromo ester). The Checkers did not monitor the reaction, but found that the product could be analyzed by GC-MS (Model HP 5890A GC equipped with an HP 5970 MS detector; column HP-1).
10.
A large portion of the aqueous solution froze on contact with the reaction mixture. The frozen material was allowed to thaw before performing the extraction.
11.
A single distillation afforded material that was contaminated by the starting bromo ester (which could be detected by NMR spectroscopy) and the brominating agent (1,2-dibromo-1,1,2,2-tetrachloroethane). The latter impurity was difficult to identify by TLC (not UV active and did not readily stain), GC (did not combust nor did it ionize easily), IR (C-Cl stretches were weak and were obscured by the dibromo ester), or NMR (no protons to observe and the
13C resonance was suppressed by the long relaxation time and the lack of a proton nOe). Combustion analysis proved to be the only reliable method to determine the purity of the distillate.
The combustion analysis results clearly demonstrated that the primary distillation did not satisfactorily remove the haloalkane. Both of these impurities were removed by a second distillation (65–67 °C, 9 mmHg) to afford 29.3–29.4 g (74–75%) of analytically pure material.
The dibromo ester was a stable compound that can be distilled, purified by silica gel column chromatography, and stored for months in a refrigerator.
12.
Physical and spectroscopic properties of
1 are as follows:
1H-NMR
pdf (500 MHz, CDCl
3) δ: 1.36 (t, 3 H,
J = 7.0), 2.65 (s, 3 H), 4.34 (q, 2 H,
J = 7.0).
13C-NMR
pdf (125 MHz, CDCl
3) δ: 13.7 (q), 37.3, 52.2, 63.7, 166.8. IR (film) cm
−1: 1737. EI-MS
m/z: 260 (M
++2), 258 (M
+). Anal. Calcd for C
5H
8Br
2O
2: C, 23.10; H, 3.10; Br, 61.48. Found: C, 22.79; H, 2.91; Br, 61.53.
13.
A solution of
tert-butyllithium in pentane was purchased from Sigma Aldrich Company and was titrated by both No-D NMR spectroscopy
5a and Gilman double titration.
5b This lithium-halogen exchange procedure for the preparation of ynolates
6 is suitable for a laboratory scale because of its convenience and simplicity. Reductive lithiation with lithium metal catalyzed by naphthalene was better for larger scale.
714.
The exothermicity of the reaction subsided substantially after one half of the alkyllithium reagent was added. Thus, the rate of addition was increased after this point without a concomitant increase in internal temperature.
15.
Acetyltrimethylsilane (97%) was purchased from Aldrich Chemical Company and was distilled prior to use (bp 110–116 °C).
16.
The reaction of the acylsilane with the lithium ynolate was exothermic and reached a maximum temperature of 39 °C. The addition rate was adjusted to minimize the loss of pentanes from the solution.
17.
TLC analysis was performed on Merck silica gel 60 F-254 plates eluting with hexanes/EtOAc, 4:1, R
f = 0.43 (visualized with 254 nm UV lamp).
18.
The product was obtained in analytically pure form by distillation on a half scale. The submitters reported that distillation of the product on the full scale led to polymerization.
19.
Physical and spectroscopic properties of
2 are as follows:
1H-NMR
pdf (500 MHz, CDCl
3) δ: 0.16 (s, 9 H), 1.90 (q, 3 H,
J = 1.0), 1.97 (q, 3 H,
J = 1.0), 11.6–12.6 (br s, 1 H).
13C-NMR
pdf (125 MHz, CDCl
3) δ: 0.2, 15.4, 20.4, 136.0, 155.6, 175.1.
29Si-NMR (119 MHz, CDCl
3) δ: −5.15. IR (film) cm
−1: 2966, 1679. MS (EI)
m/z 157 (M
+ -Me). Anal. Calcd. for C
8H
16O
2Si C, 55.77; H 9.36. Found: C, 55.78, H. 9.63.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Ynolates
8 are ketene anion equivalents and are expected to act as multifunctional carbanions for highly reactive ketenes. Although about 10 reports on the syntheses of ynolates have been published, studies on the versatility of ynolates have remained at a rather basic level, probably due to a lack of convenient and general methods for their synthesis. In
Organic Syntheses, only one report on ynolates has been published.
9The procedure described here demonstrates a practical and convenient method for the generation of ynolates, and a (Z)-selective olefination of acylsilanes leading to (Z)-2-methyl-3-trimethylsilyl-2-butenoic acid.
The method for the generation of ynolates involves thermal cleavage of ester dianions derived from α,α-dibromo esters. We have synthesized aliphatic dibromo esters (R = Me, Bu,
i-Pr, cyclohexyl,
t-Bu, phenylethyl, trimethylsilyl, etc.) by the method described herein. Aromatic dibromo esters are synthesized via radical bromination. Two procedures for the generation of ester dianions from the dibromo esters have been developed. Herein, lithium-halogen exchange of dibromo esters via
tert-butyllithium giving the ester dianions is described. This procedure is very convenient, simple and suitable for laboratory scale. In an alternative procedure, the naphthalene-catalyzed reductive lithiation
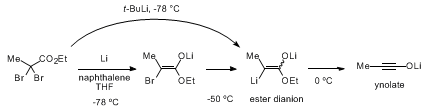
6 of the dibromo esters with lithium metal is employed. As outlined in the following scheme of for the generation of ynolates using reductive lithiation catalyzed by naphthalene,
10 the dibromo ester reacts with lithium naphthalenide at −78 °C to give an α-bromo ester enolate, which is successively lithiated to form the ester dianion. The dianion is cleaved at 0 °C to generate an ynolate by release of lithium ethoxide.
Vinylsilanes are powerful tools in synthetic organic chemistry. Although olefination of acylsilanes is expected to be a useful method for the preparation of vinylsilanes, few reports on this process have been reported due to the unsuitability of conventional olefination reagents. We have developed a method in which olefination of ketones via ynolates provides α,β-unsaturated carboxylates with good to moderate
E/Z selectivities.
11 Using acylsilanes as a ketone in this process, however, extremely high
Z-selectivity is achieved in the synthesis of various substituted vinylsilanes. The
E/Z selectivity is controlled in the electrocyclic ring-opening of the intermediate β-lactone enolates, prepared by cycloaddition of carbonyl groups with ynolates. In this step, the transition state would be stabilized by the orbital interaction between a breaking C-O s orbital and Si vacant orbitals.
12The vinylsilanes produced can be converted into a variety of functionalized multisubstituted alkenes.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1,2-Dibromo-1,1,2,2-tetrachloroethane; (630-25-1)
Ethyl 2-bromopropanoate:
Propanoic acid, 2-bromo-, ethyl ester: (535-11-5)
Ethyl 2,2-dibromopropionate:
Propanoic acid, 2,2-dibromo-, ethyl ester; (34757-17-0)
Acetyltrimethylsilane; (13411-48-8)
 |
Mitsuru Shindo was born in Tokyo in 1963. He received his PhD degree at University of Tokyo under the supervision of the late Professor K. Koga. From 1990 until 1996, he was an assistant professor at the laboratory of Professor K. Koga. He spent time in Professor R. A. Holton's group (Florida State University) as a postdoctoral fellow from 1992 until 1994. In 1996, he moved to the University of Tokushima as an associate professor working with Professor K. Shishido. In 2005, he joined the faculty at Kyushu University (Prof. Kanemasa's group) as an associate professor. He is interested in finding new methodologies for synthetic reactions and in the total synthesis of biologically important natural products.
|
 |
Kenji Matsumoto was born in Hiroshima (Japan) in 1978. He graduated from the University of Tokushima in 2001, where he received his Ph.D. in Organic Chemistry in 2006 under the supervision of Professors Mitsuru Shindo and Kozo Shishido. From 2003 until 2006, he was a research fellow of the Japan Society for the Promotion of Science (JSPS). Since 2006 he has worked as a postdoctoral fellow under the direction of Professor Sergey A. Kozmin at the University of Chicago (USA). He is interested in the development of useful synthetic methods and efficient synthetic strategies.
|
 |
Kozo Shishido received his Ph.D degree from Tohoku University in 1976 under the direction of the late Professor Tetsuji Kametani. After he was appointed as Assistant Professor at Tohoku University, he had postdoctoral fellowships with Professor A. I. Scott (Texas A&M University) and Professor M. E. Jung (UCLA) from 1978 to 1980. He then moved to University of Tokushima as Associate Professor in 1989. Since 1994, he has been a Full Professor at the Graduate School of Pharmaceutical Sciences, University of Tokushima. His main research interests are devoted to total synthesis of biologically active natural products and development of new preparative methods.
|
 |
Brian Eklov graduated with a B.S. in Chemistry from the University of Michigan in 1998 and completed his Ph.D. thesis in 2005 under the direction of Professor Thomas. R. Hoye at the University of Minnesota. His thesis included discussions of a hybrid computational/ 1H NMR based method for the elucidation of the relative configuration of complex organic molecules, developing the use of non-deuterated solvents for 1H NMR spectroscopy (No-D NMR), and a total synthesis of (+)-gigantecin, a skipped bis-THF acetogenin. He is presently pursuing post-doctoral research with Professor Scott Denmark at the University of Illinois.
|
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved