Org. Synth. 2010, 87, 161
DOI: 10.15227/orgsyn.087.0161
CYCLOHEXENE IMINE
[7-AZA-BICYCLO[4.1.0]HEPTANE]
Submitted by Iain D. G. Watson, Nicholas Afagh, and Andrei K. Yudin
1.
Checked by Lars Troendlin and Andreas Pfaltz.
1. Procedure
WARNING: Organic azides can be unstable and/or explosive.
A. trans-2-Azido-cyclohexanol. In a 500-mL, one-necked, round-bottomed flask equipped with a magnetic stir bar (4 cm, cylindrical-shaped) is placed sodium azide (25.2 g, 388 mmol, 2.5 equiv) (Note 1). Water (85 mL) is added and the solution is stirred at room temperature until all solid has dissolved. Acetone (60 mL) is added followed by a slow and continuous dropwise addition of cyclohexene oxide (15.5 mL, 153 mmol, 1.0 equiv) in acetone (25 mL) using a pressure equalizing dropping funnel over 30 min (Note 2). The flask is fitted with a reflux condenser and the solution is heated to reflux for 18 h (85 ºC), after which acetone is evaporated in vacuo and the residual solution is extracted with diethyl ether (3 × 200 mL). The combined organic phases are washed with brine (100 mL) and dried over Na2SO4. The solvent is removed in vacuo (Note 3) and trans-2-azido-cyclohexanol is obtained in 99% yield as a yellow oil (21.6 g, 153 mmol), which is reacted immediately and without further purification (Note 4).
B. Cyclohexene imine. Into a flame-dried, 500-mL, three-necked, round bottomed flask equipped with a magnetic stir bar (4 cm, cylindrical-shaped), reflux condenser, a pressure equalizing dropping funnel, internal thermometer and high-vacuum-argon connection is added the azido alcohol (21.6 g, 153 mmol, 1.0 equiv) followed by freshly distilled methyl tert-butyl ether (MTBE) (40 mL) (Note 5). Triphenylphosphine (40.2 g, 153 mmol, 1.0 equiv) is dissolved in MTBE (100 mL) and the solution is transferred into the dropping funnel. The solution is added slowly and continuously over 45 min (Note 6). The addition is accompanied by the vigorous evolution of nitrogen gas and a temperature increase to 40 °C. After the addition of the triphenylphosphine solution, the dropping funnel is washed with TBME (20 mL). After nitrogen evolution has ceased, the solution is heated to reflux (70 ºC) for 16 h under a nitrogen atmosphere. The solution is then transferred in a well ventilated hood to a single-necked, 1-L evaporating flask (Note 7). MTBE is removed at 40 ºC under reduced pressure (360 mmHg) on a rotary evaporator (Note 8) leaving an off-white solid (Ph3PO), within which is trapped the cyclohexene imine product. The cyclohexene imine is distilled in vacuo (0.01 mmHg) at 160 ºC from the resulting residue using a short path distillation apparatus to afford a clear, colorless liquid (Note 9). Residual MTBE, which will inevitably co-distill with the product, can be removed by freeze-drying the resulting liquid. In this procedure, the flask containing the cyclohexene imine is immersed in a -12 ºC sodium chloride/ice cold bath and the flask is rotated such that as cyclohexene imine begins to solidify, it forms a thin coat along the interior of the flask effectively maximizing the surface area and facilitating removal of MTBE. While still immersed in the cold-bath, a spatula is used to scratch the sides of the flask to break up the thin layer of cyclohexene imine to afford fine flakes. The residual MTBE is then removed in vacuo (0.13 mbar) while the flask remains immersed in the cold-bath to afford pure cyclohexene imine (13.6 g, 140 mmol) in 91% yield as fine white crystals below 5 ºC and a clear colorless liquid above this temperature (Notes 10 and 11; for GC data, see Note 12).
2. Notes
1.
All reagents were purchased from Sigma-Aldrich. MTBE was purchased from Sigma-Aldrich; all other solvents were purchased from EMD Chemicals. Sodium azide must be measured with a plastic or glass spoon. Do not allow sodium azide to come in contact with metal or chlorinated solvents.
2.
If cyclohexene oxide is added neat, all at once, a white insoluble polymeric material is formed which is deposited on the side of the flask, negatively affecting the isolated yield.
3.
It is important that the product is dried as water will negatively affect subsequent synthetic manipulations. After isolation using the rotary evaporator, residual water is removed from the product using a vacuum pump (0.1 mmHg).
4.
WARNING: organic azides can be unstable and/or explosive. 1H NMR
pdf (CDCl
3, 400 MHz) δ: 1.23-1.39 (m, 4 H), 1.71-1.78 (m, 2 H), 1.99-2.08 (m, 2 H), 2.31 (bs, 1 H), 3.15-3.21 (m, 1 H), 3.35-3.40 (m, 1 H);
13C NMR
pdf (CDCl
3, 100 MHz) δ: 23.8, 24.2, 29.7, 33.0, 67.1, 73.6. If desired, the intermediate
trans-2-azido-cyclohexanol can be further purified by flash chromatography on SiO
2 (R
f = 0.15, 95:5 hexanes/EtOAc).
5.
Anhydrous MTBE was obtained by distillation over 4Å molecular sieves (30 g for 300 mL of MTBE).
6.
The slow, continuous addition of triphenylphosphine under an atmosphere of N
2 was found to be essential in obtaining high yields. The submitters added solid triphenylphosphine using a pressure-equalizing powder addition funnel.
7.
Transferring the solution to the larger 1 L evaporating flask avoids complications arising from bumping of the residual solid during the two distillation steps.
8.
The checkers used a rotary evaporator for removing the solvent. The submitters performed the distillation in a short path apparatus. The connector between the flasks should have a sharp angle to prevent bumped triphenylphosphine oxide from contaminating the collected product. A piece of glass wool may be optionally inserted into the connector to avoid contamination of the collected product with bumped impurities. The collecting flask must be
completely immersed in dry ice/acetone to prevent loss of volatile cyclohexene imine.
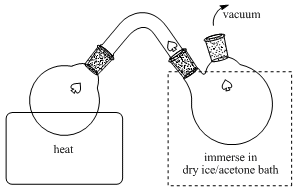
9.
Due to the low melting point of cyclohexene imine, it will occasionally freeze in the condenser (indicated by the arrow) at which point the distillation will arrest. If this occurs, then the vacuum can be temporarily removed and the water flowing through the condenser can be shut off and the condenser area gently heated with a heat-gun to melt the frozen product into the collecting flask. This opportunity can also be used to break up the solid in the evaporating flask using a metal spatula.
10.
Cyclohexene imine is stored over KOH pellets (4 pellets for 13.6 g of product) at -20 °C. Aziridines should always be considered as highly toxic compounds and treated with care in ventilated spaces.
11.
1H NMR
pdf (CDCl
3, 400 MHz) δ: 0.41 (br, 1 H), 1.16-1.36 (m, 4 H), 1.77 (s, 4 H), 2.14 (s, 2 H), 3.21 (d,
J = 2 Hz);
13C NMR
pdf (CDCl
3, 100 MHz) δ: 20.4, 24.5, 29.2. MS (ESI)
m/z (rel. intensity) 196 ([2M]
+, 75), 98 ([M+1]
+, 100), 65 (30).
12.
GC analysis was conducted immediately after distillation of cyclohexene imine. A sample of cyclohexene imine was dissolved in acetonitrile (HPLC grade) and subjected to GC analysis, which revealed the presence of 4 peaks (relative to MeCN blank). The major peak (
tR = 9.750 min) accounted for 98.9% of the total area of all peaks. Gas-phase chromatography was performed on an HP-6890 series instrument using an HP-5 column (crosslinked 5% phenyl methyl siloxane, 30 m × 0.32 mm × 0.25 μm film thickness). The oven was heated at 30 °C for 5 min followed by a temperature gradient of 5 °C/min to 120 °C. Inlet temperature and pressure were 200 °C and 4.88 psi respectively, with a split ratio of 50:1. Hydrogen was the carrier gas. The checkers used a Carlo Erba Instrument GC 8000Top, an Restek Rtx-1701 column (14% cyanpropylphenyl - 86% dimethylpolysiloxan, 30 m × 0.25 mm × 0.25 μm film thickness). A sample of cyclohexene imine was dissolved in TBME. The oven temperature was kept at 80 °C for 5 min followed by a temperature gradient of 5 °C/min to 130 °C and then 10 °C/min to 250 °C and, finally, at 250 °C for 5 min. Inlet temperature was 220 °C and the pressure was 60 kPa, helium was the carrier gas. The major peak (
tR = 10.64 min) accounted for 96.6% of the total area of all peaks.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
N-Unsubstituted, or NH-aziridines have been used as key substrates in a number of different reactions over the course of our work in this area.
2 These substrates may be prepared in a number of different ways: by Staudinger, Wenker, Gabriel or conjugate addition strategies.
3 Starting materials for NH-aziridines may include epoxides, aminoalcohols, or α,β-unsubstituted carbonyl compounds.
Over the course of the work on the functionalization of aziridines, cyclohexene imine was used extensively as a test substrate (Figure 1). This molecule contains several favorable properties, including relative ease of preparation. It can be distilled below 60 ºC under reduced pressure allowing for easy purification. The material is not volatile enough to evaporate while heating over the course of long reaction times. This aziridine is also less likely to open than monosubstituted aziridines, allowing the use of harsher reaction conditions without loss of selectivity from background ring-opening of starting materials or products.
Figure 1. Cyclohexene imine as a precursor for a wide variety of nitrogen containing functionalities.
The synthesis of cyclohexene imine was accomplished by a two-step protocol.
4 First of all, the ring opening of cyclohexene oxide with sodium azide provided the azidoalcohol intermediate in quantitative yield. This intermediate, isolated by extraction, was then reacted without further purification by the addition of one equivalent of triphenylphosphine.
Figure 2. Mechanism of the Staudinger reaction, formation of cyclohexene imine.
The second step of the protocol, the Staudinger reaction, proceeds via initial reduction of the azide group to generate a five-membered intermediate (Figure 2). Decomposition of the intermediate then generates the aziridine by exclusion of triphenylphosphine oxide. Reduction of the azide group occurs immediately upon addition of triphenylphosphine, evident by bubbling caused by the evolution of nitrogen gas. However, heating the solution is necessary for the production of the aziridine. In fact, when the reaction was performed in diethyl ether, yields were much lower than when THF or methyl tert-butyl ether were used, owing to the higher boiling points of the latter solvents.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Cyclohexene oxide, 98%; (286-20-4)
Triphenylphosphine, 99%; (603-35-0)
Sodium azide, ≥99.5%; (26628-22-8)
 |
Professor Andrei K. Yudin obtained his B.Sc. degree at Moscow State University and his Ph.D. degree at the University of Southern California under the direction of Professors G. K. Surya Prakash and George A. Olah. He subsequently took up a postdoctoral position in the laboratory of Professor K. Barry Sharpless at the Scripps Research Institute. In 1998, he started his independent career at the University of Toronto. He received early tenure in 2002 and became Full Professor in 2007. He is a recipient of a number of awards and his research interests are in development and application of novel synthetic methods that enable discovery of functionally significant molecules.
|
 |
Iain Watson was born in Toronto, Canada. He is a medicinal chemist at the Ontario Institute for Cancer Research (OICR) in Toronto. Iain worked on the development of asymmetric gold(I)-catalyzed cycloisomerization reactions during his postdoctoral studies at the University of California, Berkeley with Professor F. Dean Toste. He received his Ph.D. from the University of Toronto in 2006, working with Professor Andrei Yudin in the fields of amination and palladium catalysis.
|
 |
Nicholas A. Afagh received a B.Sc. in biochemistry from the University of Ottawa in 2007, working under the supervision of Prof. Robert N. Ben. He is currently a graduate student in the group of Professor Andrei K. Yudin at the University of Toronto, where he is investigating the synthetic applications of N-alkenyl aziridines.
|
 |
Lars Tröndlin was born in 1977 in Lörrach, Germany. He studied Chemistry at the University of Basel where he obtained his M.Sc. in 2006 under the supervision of Prof. Andreas Pfaltz. He began his Ph.D. work in summer 2006 in the group of Prof. Andreas Pfaltz, where he is currently working on synthesis of new chiral ligands for metal-catalyzed reactions.
|
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved