Org. Synth. 2011, 88, 70-78
DOI: 10.15227/orgsyn.088.0070
Discussion Addendum for: ASYMMETRIC SYNTHESIS OF (M)-2-HYDROXYMETHYL-1-(2-HYDROXY-4,6-DIMETHYLPHENYL)NAPHTHALENE VIA A CONFIGURATIONALLY UNSTABLE BIARYL LACTONE
Submitted by G. Bringmann
1, T.A.M. Gulder, and T. Gulde.
Discussion
The 'lactone concept' has in recent years been used for the atroposelective construction of a broad variety of axially chiral natural products,
2 especially for the synthesis of a large number of naphthylisoquinoline alkaloids with different coupling positions, substitution patterns, and oxidation states in the respective isoquinoline portions.
3An instructive example of the asymmetric synthesis of a representative of this group of alkaloids is the atropo-diastereodivergent construction of the two epimers korupensamine A [(P)-
7] and B [(M)-
7] from the same late precursor
4 (Scheme 1).
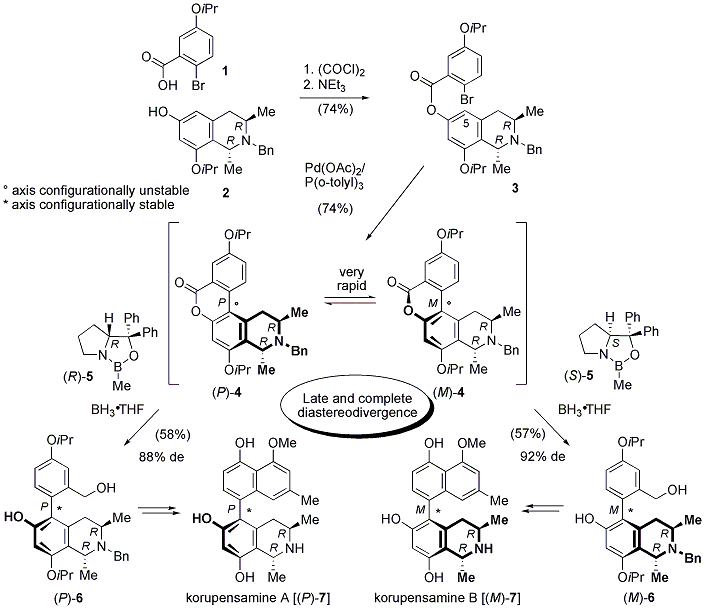
4 The two required building blocks, the readily available carboxylic acid
15 and the optically pure tetrahydroisoquinoline
2,
6 were linked together by esterification giving
3 in 74% yield. Pd-catalyzed intramolecular cross-coupling reaction of
3 using the 'Herrmann-Beller' catalyst
7 delivered the very rapidly interconverting biaryl lactones (P)- and (M)-
4 in 74% yield. The dynamic kinetic resolution by reductive ring cleavage of
4 proceeded highly atroposelectively when applying borane activated by
5. Utilization of (S)-
5 afforded the ring-opened and therefore configurationally stable benzylic alcohol (M)-
6 in 58% yield and an excellent 92% de, while the analogous reaction with the enantiomeric reagent (R)-
5 diastereoselectively gave the epimer (P)-
6, yet with a slightly lower asymmetric induction (88% de). The synthesis of both, (P)-
7 and (M)-
7, was completed by using exactly the same reaction conditions starting from
6 in 10 steps and ca. 10% overall yield.
4Scheme 1
Besides providing an atropo-diastereodivergent synthetic route to naphthylisoquinoline alkaloids, the 'lactone concept' paved the way for the synthesis of many other biaryl systems, like e.g. the phenylanthraquinone knipholone [(P)-
14].
8 In this case, the key precursor
10 was obtained by esterification of the dibromide
8 with dimethoxyphenol
9 in 90% yield (Scheme 2). After pre-fixation of the two molecular portions, Pd-mediated
C-
C bond formation to give the configurationally unstable lactones
11 occurred in 68% yield, despite the large steric hindrance exerted by the presence of four substituents ortho to the biaryl axis. Dynamic kinetic resolution by atropo-enantioselective reduction utilizing (S)-
5 furnished (M)-
129 in good yield and almost perfect enantioselectivity (96% ee), which was enhanced by a simple recrystallization to obtain enantiopure material (99% ee, 65% yield). The optically less pure mother liquor was recycled by a three-step oxidative procedure regenerating
11 in 71% yield. The target molecules, 6'-
O-methylknipholone [(M)-
13] and knipholone [(P)-
14], were obtained from (M)-
12 by simple functional group conversions.
8Scheme 2
Another example of the broad applicability of the 'lactone concept' is the atroposelective synthesis of the AB-fragment of vancomycin-type glycopeptides.
10 The key intermediate, ester
17, was obtained by a simple esterification of carboxylic acid
15 with phenol
16 in good 81% yield (Scheme 3). Intramolecular Heck reaction gave the configurationally unstable lactone
18 (64%) which after reductive cleavage of the heterocyclic ring by using borane activated (S)-
5, yielded the biaryl (M)-
19 as a promising precursor for the vancomycin AB-fragment.
Scheme 3
The versatility of the 'lactone concept' has been demonstrated by our group in the total synthesis of several structurally diverse compounds, such as the bicoumarin isokotanin A [(M)-
20],
11 the biscarbazole (M)-
21,
12 which was attained by cleavage of the lactone bridge utilizing a chiral
O-nucleophile [(R)-mentholate], and the biphenol mastigophorene A [(P)-
22]
13 (Scheme 4).
Scheme 4
The 'lactone concept' for the construction of axially chiral natural products has also been used by other groups, i.a. by Abe, Harayama, et al.
14 Reductive cleavage of the configurationally unstable biaryl lactone
23 employing (S)-
5 gave (M)-
24, a precursor for the formal total synthesis of
(-)-steganone (M)-
25 (Scheme 6). The introduction of the chiral information into the biaryl system (M)-
24 was attained with excellent chemical (97%) and optical (83% ee) yields.
Scheme 5
The concept of using chiral
N-nucleophiles in the atroposelectivity determining step, as previously elaborated in our group,
15,16 was also adopted by Suzuki et al. in the synthesis of benanomicin B (
28).
17 Ring opening of the lactone system in
26 was thus achieved by using (S)-valinol to give (M)-
27 in good 90% yield and 82% de (Scheme 6). The asymmetric information of the biaryl axis was then transmitted to the stereoselective formation of the trans
-diol in the final product by a semipinacol cyclization of an intermediate acetal-aldehyde with full stereocontrol. This example demonstrates that - by the use of the 'lactone concept' - axially chiral biaryls (like
27) are now available so efficiently that they may even serve as a 'cheap' precursor for the construction of chiral target molecules with merely central chirality.
Scheme 6
The 'lactone concept' is not restricted to the dynamic kinetic resolution of biaryls that are configurationally labile due to the presence of a 6-membered lactone bridge, but can also be applied to 7-membered biaryl lactones, which are normally stable at the axis, thus just permitting a normal - non-dynamic - kinetic resolution.
3a This alternative is well suited in particular for the preparation of constitutionally symmetric biaryls, since it avoids the need of building up two different aryl compounds (cf. Scheme 7). In the synthesis of the dimeric sesquiterpenes mastigophorene A [(P)-
22] and B [(M)-
22]
18 it thus provides the as yet shortest atroposelective approach (22 steps overall) to date by an atropo-diastereomer-differentiating reduction of
30 with borane and the CBS catalyst (R)-
5 in the key step, obtaining the diol (P)-
31 with a low 30% de, whilst the unreactive lactone (M)-
31 was recovered with a better diastereomeric excess of 62%.
Scheme 7
A 7-membered lactone was also utilized by Molander et al. in the atroposelective construction of (M)-
32 in their synthesis of (+)-isoschizandrin [(M)-
35]
19 (Scheme 8). Starting from a racemic mixture of
32, the undesired atropo-enantiomer was selectively consumed by enantiomer-differentiating reduction employing (R)-
5, resulting in enantiopure unreacted lactone (M)-
33 (98% ee). The product of the reduction step, the P-configured diol (not shown), was re-oxidized and recyclized to again give
32. Applying this recycling procedure, the overall yield of (M)-
33 was improved to 61%. DIBALH mediated reduction of (M)-
33 yielded aldehyde (M)-
34, which was transformed to (+)-isoschizandrine [(M)-
35] in six further steps.
19Scheme 8
More recently, Abe et al. applied the 'lactone concept' to the enantioselective construction of a valoneic acid derivative.
20,21 In addition, Yamada et al. elaborated catalytic atropo-enantioselective versions of this method using cobalt
22 and BINAP-based AgBF
4−phosphine complexes.
23
 |
Gerhard Bringmann studied chemistry and biology in Gießen and Münster. After his Ph.D. with B. Franck in 1978, postdoctoral studies with D.H.R. Barton in Gif-sur-Yvette, and his habilitation in Münster, he received offers for full professorships at the Universities of Vienna and Würzburg, of which he accepted the latter in 1987. His research focuses on analytical, synthetic, and computational natural product chemistry, e.g., on axially chiral biaryls. He received, i.a., the Prize for Good Teaching (1999), the Adolf-Windaus Medal (2006), the Honorary Doctorate of the University of Kinshasa (2006), the Paul-J.-Scheuer Award (2007), and the Honorary Guest Professorship of Peking University (2008). |
 |
Tobias A. M. Gulder studied Chemistry at the University of Würzburg until 2004, followed by a Ph.D. with Prof. G. Bringmann in analytical and synthetic natural product chemistry, for which he received the 2009 DECHEMA PhD award. In 2008, he joined Prof. B.S. Moore at Scripps Institution of Oceanography (UCSD) as a DAAD postdoctoral fellow working on the elucidation and exploitation of marine natural product biosynthetic pathways. In the middle of 2010 he returned to Germany supported by a DAAD reintegration fellowship to start his independent research at the University of Bonn. |
 |
Tanja Gulder was born in 1978 in Weißenburg i. Bay. Germany, and received her diploma in chemistry from the University of Würzburg in 2004. After earning her Ph.D. with distinction under the supervision of Prof. G. Bringmann at University of Würzburg in 2008, she pursued postdoctoral studies with Prof. P. S. Baran at The Scripps Research Institute (La Jolla, CA) focusing on the synthesis of strained natural products. In summer 2010 she started her independent career at RWTH Aachen university supported by a Liebig fellowship. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved