Org. Synth. 2012, 89, 374-379
DOI: 10.15227/orgsyn.089.0374
Discussion Addendum for:
[Trifluoromethylation at the α-Position of α,β-Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone]
Submitted by Kazuyuki Sato, Atsushi Tarui, Masaaki Omote, Itsumaro Kumadaki, and Akira Ando
1.
Discussion
Trifluoromethylated (CF3) compounds are one of the most important classes of fluorine compounds, but the reactions for introducing a CF3 group at the α-position of carbonyl compounds were rarely reported until recently.
The reductive α-trifluoromethylation of α,β-unsaturated ketones using the combination of RhCl(PPh3)3-Et2Zn, based on the Reformatsky-Honda reaction of non-fluorinated α-bromoacetate, became one of the breakthrough reactions in this field.
The birth and advances of this α-trifluoromethylation and related reactions will be summarized here.
α-Fluoroalkylation Using Reformatsky-Honda Reaction Condition
Formerly, we applied the so-called Reformatsky-Honda reaction, using RhCl(PPh
3)
3-Et
2Zn,
2 to the reaction of 2-cyclohexen-1-one with ethyl bromodifluoroacetate (BrCF
2COOEt), and found that the reaction afforded either the α-(ethoxycarbonyl)difluoromethyl ketone or the usual Reformatsky-type product selectively only by changing the solvent (Figure 1).
3 The former was a new type of product; thus, we tried to expand this reaction to the synthesis of α-fluoroalkyl compounds, especially α-CF
3 compounds.
After many efforts, we succeeded in the synthesis of α-CF
3 and other fluoroalkyl (R
f) ketones from α,β-unsaturated ketones,
4,5 and showed through the use of Et
2Zn-
d10 that a Rh-H complex played an important role (Figure 2).
5 Furthermore, based on this mechanism, we developed a new methodology for synthesis of α-R
f ketones from silyl enol ethers of ketones in the absence of Et
2Zn, although this reaction required high temperature.
6 The addition of Et
2Zn allowed the reaction to proceed at lower temperature, improved the yield
7, and extended its application to various fluoroalkyl halides (Figure 3).
8 Formation of the Rh(I)Et complex appears to promte the oxidative addition of CF
3I at lower temperature.
Figure 1. Solvent effect of Rh-catalyzed reaction of BrCF2COOEt with 2-cyclohexen-1-one.
Figure 2. Rh-Catalyzed reductive α-fluoroalkylation and its mechanism.
Figure 3. Synthesis of various α-fluoroalkylated carbonyl compounds.
The Reformatsky-Honda Reaction of BrCF2COOEt with Imines
This Reformatsky-Honda reaction of BrCF
2COOEt has been applied to imines.
Interestingly, we succeeded in selective syntheses of difluoro-β-lactams and 3-amino-2,2-difluorocarboxylic esters (Figure 4), depending upon the presence or absence of MgSO
4·7H
2O.
9 MgSO
4·7H
2O serves as a convenient source of H
2O.
Addition of H
2O also provided the ester, but the yield was lower.
Furthermore, highly stereoselective syntheses of difluoro-β-lactams have also been accomplished using chiral auxiliaries.
10Figure 4. Reformatsky-Honda reaction of BrCF2COOEt with imines.
Reductive Reformatsky-Honda Reaction
The mechanistic studies of α-fluoroalkylation of α,β-unsaturated ketone led to another new discovery.
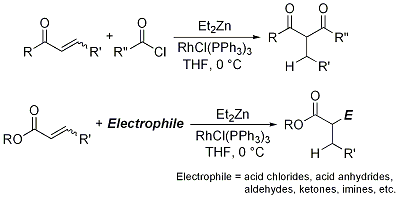
The most recent advance has been a synthesis of 1,3-diketones by reductive α-acylation of α,β-unsaturated ketones under the Reformatsky-Honda reaction conditions that easily generate a Rh-H complex (Figure 5).
11 α,β-Unsaturated esters can be utilized with various electrophiles besides acid chlorides to give reductive α-substitution products such as aldol-type and Mannich-type products.
12Figure 5. Reductive Reformatsky-Honda reaction with acid chlorides and various electrophiles.
 |
Kazuyuki Sato studied organofluorine chemistry at Faculty of Pharmaceutical Sciences, Setsunan University.
He received Ph.D.
degree from Setsunan University on the study of synthesis of fluorine compounds using cupper-mediated reactions of ethyl bromodifluoroacetate under the guidance of Prof.
Itsumaro Kumadaki in 2003.
Since 1999, he is working as a research associate at Setsunan University.
His main interest is rhodium-catalyzed synthesis of fluorine compounds.
He received two awards from the Pharmaceutical Society of Japan and the Society of Synthetic Organic Chemistry, Japan. |
 |
Atsushi Tarui was born in 1981 in Wakayama (Japan).
He received Ph.D.
degree from Setsunan University on the research of synthesis of α,α-difluoro-β-lactam using rhodium-catalyzed reaction of bromodifluoroacetate with imines and related reactions under supervision of Prof.
Akira Ando in 2010.
Since 2006, he is working there as a research associate under the guidance of Prof.
A.
Ando.
His research interest is synthesis of fluorinated β-lactams and β-amino acids. |
 |
Masaaki Omote received his Ph.D.
from Faculty of Pharmaceutical Sciences, Setsunan University under the supervision of Prof.
Itsumaro Kumadaki in 1997, and he became research associate of Setsunan University.
In 2004, he spent a year as a postdoctoral research associate in State University of New York (Albany, New York) under the supervision of Prof.
John T.
Welch.
In 2010 he promoted to associate professor of Setsunan University.
His research interest is syntheses of fluorous and partially fluorinated chiral ligands useful for enantioselective synthesis of non-fluorinated compounds. |
 |
Itsumaro Kumadaki received Ph.D.
degree from Kyushu University on the study of reaction of heteroaromatic amine N-oxides under the supervision of Prof.
Masatomo Hamana in 1968.
He joined Prof.
Yoshiro Kobayashi of Tokyo College of Pharmacy in 1967 and started the studies on organic fluorine chemistry.
He moved to Setsunan University in 1983 as a professor of Faculty of Pharmaceutical Sciences, and retired in 2006.
His interests are still new reactions for synthesis of organofluorine compounds, new reactions of organofluorine compounds, and chemical and biological effects of fluorine substituents on organic compounds. |
 |
Akira Ando was born in 1951 in Tokyo (Japan), and obtained Ph.D.
degree from Tokyo College of Pharmacy on the study of the synthesis and reactions of valence-bond isomers of tetrakis(trifluoromethyl)pyrroles under supervision of Prof.
Yoshiro Kobayashi in 1980.
After working as a research assistant at Tokyo College of Pharmaceutical Sciences and a research fellow at Sagami Chemical Research Center, he joined Prof.
Kumadaki's group of fluorine chemistry at Setsunan University in 1985, and since 2004 he is a full professor there.
His interest is to develop new methodologies for synthesis of organofluorine compounds. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved