Org. Synth. 2021, 98, 263-288
DOI: 10.15227/orgsyn.098.0263
Preparation of Hindered Aniline CyanH and Application in the Allyl-Ni-Catalyzed α,β-Dehydrogenation of Carbonyls
Submitted by Alexandra K. Bodnar, Aneta Turlik, David Huang, Will Butcher, Joanna K. Lew, and Timothy R. Newhouse*
1Checked by Aoi Takeuchi, Hiroaki Itoh, and Masayuki Inoue
1. Procedure (Note 1)
A. CyanH (N-cyclohexyl-2,6-diisopropylaniline) (1). An oven-dried, 1-L 3-necked round-bottomed flask (central neck 29/32 joint, side necks 15/25 joint) is equipped with a 4.5 cm x 2 cm Teflon-coated stir bar. One side neck is fitted with a 15/25 rubber septum with argon inlet and outlet needles, and the other is fitted with a 100-mL addition funnel (15/25 joint). The central neck is fitted with a 29/42 rubber septum (Figure 1). After the substitution of the gas by an argon flow, the rubber septum is removed from the central neck of the flask. NaBH4 (17.5 g, 463 mmol, 3.0 equiv) (Note 2) and 1,2-dichloroethane (300 mL, 0.5 M) (Note 3) are added through the central neck (Figure 2A). Then, the central neck is fitted with the 29/42 rubber septum again. The white cloudy suspension is cooled to 0 °C in an ice-water bath for 10 min. Acetic acid (80 mL, 1400 mmol, 9.0 equiv) (Note 4) is added to the mixture via addition funnel over 25 min with vigorous H2 gas evolution (Figure 2B). After the H2 evolution ceases, the resulting white cloudy suspension is stirred for an additional 30 min at 0 °C. 2,6-Diisopropylaniline (29 mL, 154 mmol, 1.0 equiv) (Note 5) is added dropwise via addition funnel over 5 min, followed by the addition of cyclohexanone (32 mL, 308 mmol, 2.0 equiv) (Note 6) dropwise via addition funnel over 5 min. The addition funnel is replaced with a 15/25 rubber septum (Figure 2C). The resulting white cloudy solution is placed in a pre-heated 35 °C oil bath and is stirred for 40 h (500 rpm) (Note 7).

Figure 1. Reaction set-up photo (photo provided by checkers)
Figure 2. Reaction after addition of A) sodium borohydride, B) acetic acid, and C) 2,6-diisopropylaniline and cyclohexanone and removal of the addition funnel (photos provided by checkers)
A 2.5 M aqueous NaOH solution (400 mL) (Note 8) is slowly added to the reaction flask, which warms upon quenching over 10 min (Figure 3). The mixture is stirred for an additional 10 min. The warm mixture is then cooled in an ice-water bath for 30 min and subsequently transferred to a 1- L separatory funnel, and the reaction vessel is rinsed with EtOAc and water. The organic layer is separated, and the aqueous layer is extracted with EtOAc (3 x 100 mL). The 1,2-dichloroethane and EtOAc layers are combined, washed with brine (250 mL), dried over anhydrous Na2SO4 (70 g) (Note 9), filtered through a glass funnel equipped with cotton and concentrated under reduced pressure (100 mmHg, 36 °C) by rotary evaporation to provide 49.2 g of crude material as a viscous light pink oil (Figure 4). The product is purified by flash column chromatography on silica gel (Note 10) using 5% EtOAc in hexane as eluent (Note 11). The fractions containing the product are combined and concentrated under reduced pressure (100 mmHg, 36 °C) by rotary evaporation to provide CyanH (1) as white crystals (21.8 g, 55%) (Note 12) (Figure 5).

Figure 3. Reaction mixture after quenching with 2.5 M NaOH (photo provided by checkers)
Figure 4. Crude material after concentration under reduced pressure, as a viscous, light pink oil (photo provided by checkers)
Figure 5. Purified Product 1 (photo provided by checkers)
B. N,N-Dibenzyl-3-methylbutanamide (2). To an oven-dried 300-mL round-bottomed flask (29/42 joint) equipped with a 4.5 cm x 2 cm Teflon-coated stir bar and a 29/42 rubber septum with argon inlet and outlet needles (Figure 6) are added isovaleryl chloride (2.6 mL, 21 mmol, 1.0 equiv) (Note 13) and anhydrous diethyl ether (100 mL, 0.2 M) (Note 14) by a syringe through the rubber septum. The reaction vessel is moved to a 0 °C ice-water bath and stirred for 10 min. Triethylamine (8.9 mL, 64 mmol, 3.0 equiv) (Note 15) is added to the mixture by a syringe through the rubber septum over 3 min, resulting in a cloudy white suspension (Figure 7A). To the mixture is added dibenzylamine (Note 16) (4.5 mL, 23 mmol, 1.1 equiv) dropwise by a syringe through the rubber septum over 5 min, and the reaction is stirred for an additional hour at 0 °C (Note 17) (Figure 7B).
Figure 6. Reaction set-up (photo provided by checkers)
Figure 7. Reaction after addition of A) triethylamine and B) dibenzylamine (photos provided by checkers)
The reaction is quenched at 0 °C by the addition of a saturated aqueous solution of NH4Cl (100 mL) over 5 min (Note 18). The reaction mixture is transferred to a 500 mL separatory funnel and the organic layer is separated. The aqueous phase is extracted with diethyl ether (2 x 100 mL), and the combined organic extracts are washed with brine (100 mL), dried over anhydrous Na2SO4 (30 g), filtered through a glass funnel fitted with a cotton plug and concentrated under reduced pressure (100 mmHg, 36 °C) by rotary evaporation to provide 6.5 g of a light yellow cloudy oil (Figure 8A). The product is purified by flash column chromatography on silica gel (Note 10) using 13% EtOAc in hexane as eluent (Note 19). The fractions containing the product are combined and concentrated under reduced pressure (100 mmHg, 36 °C) by rotary evaporation to afford N,N-dibenzyl-3-methylbutanamide (2) as a light yellow oil (5.6 g, 94%) (Note 20) (Figure 8B).

Figure 8. A) Crude material as a light yellow cloudy oil. B) Dried product 2 (photos provided by checkers)
C. N,N-Dibenzyl-3-methylbut-2-enamide (3). An oven-dried, 1-L 3-necked round-bottomed flask (central neck 29/32 joint, side necks 15/25 joint) is equipped with a 4.5 cm x 2 cm Teflon-coated stir bar. One side neck is fitted with a 15/25 rubber septum, and the other is fitted with a 50-mL addition funnel (15/25 joint), which is topped with a 15/25 rubber septum. The central neck is equipped with a connecting adapter (upper outer joint 15/25, lower inner joint 29/42) and a water-cooled reflux Dimroth condenser (15/25 joint), which is topped with a 15/25 three-way stopcock connected to an argon inlet and a vacuum line. All junctures of the glassware and the rubber septa are sealed with silicone grease and Teflon tape. The rubber septum of the side neck of the flask is removed, and CyanH (1) (6.5 g, 25 mmol, 1.3 equiv) (Note 21) is added through the side neck. The rubber septum is reattached and sealed with Teflon tape. The flask is connected to the vacuum line for 2 min, after which the flask is backfilled with argon. This substitution of the gas is repeated three times (Figure 9).
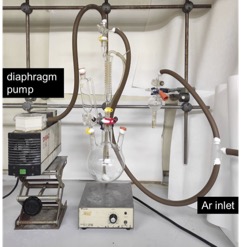
Figure 9. Reaction set up after addition of CyanH (1) (photo provided by checkers)
Tetrahydrofuran (THF, 250 mL, 0.1 M) (Note 22) is added to the flask by a syringe through a rubber septum on the side neck. The clear colorless solution is cooled to -45 °C (dry ice in acetonitrile) (Figure 10A) for 1 h. To the solution is added n-butyllithium (9.2 mL of 2.6 M in hexane (checker), 24 mmol, 1.25 equiv) (Note 23) dropwise by a syringe through a rubber septum over 5 min, resulting in a light yellow heterogeneous mixture (Figure 10B). The mixture is stirred for an additional 1.5 h at -45 °C. To the mixture is added a solution of N,N-dibenzyl-3-methylbutanamide (2) (5.4 g, 19 mmol, 1.0 equiv) (Note 24) in THF (50 mL, 0.4 M) dropwise via addition funnel over 15 min. The reaction vessel is moved to a 0 °C ice-water bath and stirred for 30 min, resulting in an orange homogeneous solution (Figure 10C).
To an oven-dried 100-mL round-bottomed flask (15/25 joint), equipped with a 2.0 x 0.9 cm Teflon-coated stir bar, is added ZnBr2 (8.6 g, 38 mmol, 2.0 equiv) (Note 25). The neck of flask is fitted with a 15/25 rubber septum with argon inlet and outlet needles. THF (40 mL, 1.0 M) is added to the flask by a syringe through the rubber septum over 1 minute. The mixture is stirred at room temperature until complete dissolution of ZnBr2 resulting in a clear, colorless solution. This ZnBr2 solution is then transferred to the addition funnel by a syringe through the rubber septum and added to the reaction mixture via addition funnel over 5 min. The reaction is stirred for an additional 30 min at 0 °C, resulting in a light yellow homogeneous solution (Figure 10D).
To an oven-dried 100-mL round-bottomed flask (15/25 joint), equipped with a 2.0 x 0.9 cm Teflon-coated stir bar, is added NiBr2(dme) (590 mg, 1.9 mmol, 0.1 equiv) (Note 26). The neck of the flask is fitted with a 15/25 rubber septum with argon inlet and outlet needles. THF (40 mL, 0.6 M) and diethyl allyl phosphate (4.1 mL, 23 mmol, 1.2 equiv) (Note 27) are added to the flask via syringe over 1 min, resulting in a deep purple homogeneous solution (Figure 10E). This solution is stirred at room temperature for 10 min and then transferred to the addition funnel by a syringe through the rubber septum (Note 28). The solution is subsequently added to the reaction mixture via addition funnel over 5 min. The reaction vessel is then placed in a pre-heated 80 °C oil bath and stirred at reflux for 4 h (Figure 10F) (Note 29).

Figure 10. A) Reaction after addition of CyanH (1) and THF and cooling to -45 °C, B) after LiCyan formation, C) after addition of 2, D) after ZnBr2 addition. E) Premixed solution of NiBr2(dme) and diethyl allyl phosphate. F) Reaction mixture during reflux (photos provided by checkers)
The reaction vessel is removed from the oil bath and allowed to return to room temperature over 30 min. The reaction is then quenched by the addition of saturated aqueous NH4Cl (500 mL) over 3 min. The quenched reaction mixture is transferred to a 2-L separatory funnel, and the reaction vessel is rinsed with EtOAc. The organic layer is separated, and the aqueous layer is extracted with EtOAc (3 x 250 mL). The combined organic layers are washed with brine (250 mL), dried over anhydrous Na2SO4 (50 g), filtered through a glass funnel equipped with cotton, and concentrated under reduced pressure (100 mmHg, 36 °C) by rotary evaporation to provide 13 g of crude material as a viscous orange oil. The product is purified by flash column chromatography on silica gel (Note 10) using a gradient of 7% EtOAc in hexane to 15% EtOAc in hexane as eluent (Note 30). The fractions containing the product are combined and concentrated under reduced pressure (100 mmHg, 36 °C) by rotary evaporation to afford N,N-dibenzyl-3-methylbut-2-enamide (3) as a slightly yellow solid (4.8 g, 89%) (Note 31) (Figure 11).

Figure 11. Dried product 3 (photo provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
isovaleryl chloride,
n-butyllithium,
triethylamine,
dibenzylamine,
zinc bromide,
diethyl allyl phosphate,
nickel (II) bromide dimethoxyethane adduct,
N-Cyclohexyl-2,6-diisopropylaniline,
2,6-diisopropylaniline,
cyclohexanone,
sodium borohydride,
acetic acid,
ethyl acetate,
hexane,
diethyl ether,
tetrahydrofuran,
1,2-dichloroethane, and
methanol, as well as the proper procedures for working with and quenching pyrophoric materials.
2.
Sodium borohydride (>98%) was obtained from Sigma-Aldrich and was used as received (checkers).
Sodium borohydride (98%) was obtained from AK Scientific and was used as received (submitters).
3.
1,2-Dichloroethane (anhydrous, 99.8%) was obtained from Sigma-Aldrich and was used as received.
4.
Acetic acid (>99.5%) was obtained from TCI and was used as received (checkers).
Acetic acid (>99.5%) was obtained from J.T. Baker and was used as received (submitters).
5.
2,6-Diisopropylaniline (90%) was obtained from TCI and was used as received.
6.
Cyclohexanone (>99.0%) was obtained from TCI and was used as received (checkers).
Cyclohexanone (99.8%) was obtained from Acros and was used as received (submitters).
7. In the TLC analysis of the crude product, the R
f value of product
1 in 25%
Et2O in
hexane was 0.74. The spot of the product on the silica gel plate [TLC silica gel 60 F
254, purchased from Merck KGaA (checkers)] was visualized under UV light (254 nm) and then stained with phosphomolybdic acid [FUJIFILM Wako Pure Chemical Corporation (checkers)] (Figure 12).
Figure 12. TLC analysis of the crude product in step A (photo provided by checkers)
8.
Sodium hydroxide (>93%) was purchased from FUJIFILM Wako Pure Chemical Corporation and was used as received (checkers).
9. Anhydrous
Na2SO4 (>99%) was purchased from Nacalai Tesque, Inc. and was used as received (checkers).
10. Silica gel (Silica gel 60N, spherical and neutral, 0.050-0.060 mm) was purchased from Kanto Chemical Co., Inc. and used as received (checkers).
11. The column with a 12 cm diameter x 50 cm height was packed with silica gel (1.08 kg) using 5%
EtOAc in
hexane (1.5 L). Then, the crude material was loaded onto the column. At that point, fraction collection (500-mL fractions) was begun, and elution was continued with 6.0 L of 5%
EtOAc in
hexane. The desired product was obtained in fractions 6-9 (Figure 13).
Figure 13. TLC analysis of column fractions in step A (photo provided by checkers)
12. Product
1 exhibited the following properties: mp = 73 °C; R
f 0.42 (5%
EtOAc in
hexane); IR (cm
-1) 2960, 2928, 2852, 1447, 1383, 1318, 1085, 795, 756;
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.11-1.22 (m, 5H), 1.25 (d,
J = 6.9 Hz, 12H), 1.65 (ad,
J = 12 Hz, 1H), 1.78 (ad,
J = 12 Hz, 2H), 2.01 (ad,
J = 12 Hz, 2H), 2.76-2.81 (m, 1H), 2.96 (br s, 1H), 3.29 (sep,
J = 6.9 Hz, 2H), 7.03-7.05 (m, 1H), 7.06-7.11 (m, 2H);
13C NMR
pdf (125 MHz, CDCl
3) δ: 24.4, 26.1, 26.2, 28.0, 34.8, 59.6, 123.0, 123.5, 142.0; HRMS (ESI)
m/z [M+H]
+ calc'd for C
18H
30N: 260.2373; found 260.2380. Purity was determined to be 98.9% by qNMR
pdf using 25.9 mg of
1 and 16.8 mg of 1,3,5-trimethoxybenzene (FUJIFILM Wako Pure Chemical Corporation, 99.8%) (checkers). When the reaction was carried out on a half-scale, 10.9 g (55%) of product
1 was obtained.
13.
Isovaleryl chloride (98%) was purchased from Sigma-Aldrich and was used as received.
14.
Diethyl ether (>99.5%) was purchased from Kanto Chemical Co., Inc. and was purified by Glass Contour solvent dispensing system (Nikko Hansen & Co., Ltd.) (checkers).
Diethyl ether was dried over 3Å molecular sieves under an atmosphere of N
2 for 48 h before use in the reaction (submitters).
15.
Triethylamine (>99.0%) was purchased from FUJIFILM Wako Pure Chemical Corporation and was distilled over CaH
2 and used immediately (checkers).
16.
Dibenzylamine (97%) was obtained from Sigma-Aldrich and was used as received.
17. In the TLC analysis of the crude product, the R
f value of product
2 in 15%
EtOAc in
hexane was 0.35. The spot of the product on the silica gel plate [TLC silica gel 60 F
254, purchased from Merck KGaA (checkers)] was visualized under UV light (254 nm) and then stained with phosphomolybdic acid [FUJIFILM Wako Pure Chemical Corp. (checkers)] (Figure 14).
Figure 14. TLC analysis of the crude product in step B (photo provided by checkers)
18.
Ammonium chloride (>98.5%) was purchased from Nacalai Tesque, Inc. and used as received (checkers).
19. The column with a 6 cm diameter x 30 cm height was packed with silica gel (137 g) using 13%
EtOAc in
hexane (150 mL). Then, the crude material was loaded onto the column. At that point, fraction collection (100-mL fractions) was begun, and elution was continued with 2.1 L of 13%
EtOAc in
hexane. The desired product was obtained in fractions 8-18 (Figure 15).
Figure 15. TLC analysis of column fractions in step B (photo provided by checkers)
20. Product
2 exhibited the following properties: R
f 0.35 (15%
EtOAc in
hexane); IR (cm
-1) 2957, 2870, 1646, 1494, 1449, 1421, 1209, 732, 692;
1H NMR
pdf (500 MHz, CDCl
3) δ: 0.98 (d,
J = 7.4 Hz, 6H), 2.23-2.31 (m, 3H), 4.46 (s, 2H), 4.61 (s, 2H), 7.16 (d,
J = 9.7 Hz, 2H), 7.23 (d,
J = 9.7 Hz, 2H), 7.27-7.33 (m, 4H), 7.36-7.40 (m, 2H);
13C NMR
pdf (125 MHz, CDCl
3) δ: 22.8, 25.9, 42.1, 48.0, 49.9, 126.4, 127.4, 127.6, 128.3, 128.6, 129.0, 136.7, 137.7, 173.1; HRMS (ESI)
m/z [M+H]
+ calc'd for C
19H
23NO
+: 282.1852; found 282.1865. Purity was determined to be 98.6% by qNMR
pdf using 22.3 mg of
2 and 13.3 mg of 1,3,5-trimethoxybenzene (FUJIFILM Wako Pure Chemical Corporation, 99.8%) (checkers). When adjusted for purity the yield is 90.5% (5.52 g). When the reaction was carried out on a half-scale, 2.8 g (94%) of product
2 was obtained.
21.
CyanH (36.2 g) was recrystallized from
methanol (90 mL) and allowed to dry under reduced pressure overnight or for at least 12 hours.
22.
Tetrahydrofuran (>99.0%) was purchased from Kanto Chemical Co., Inc. and purified by Glass Contour solvent dispensing system (Nikko Hansen & Co., Ltd.) (checkers).
23.
n-Butyllithium solution was purchased from Kanto Chemical Co., Inc. (2.6 M in
hexane) and was used as received (checkers).
n-Butyllithium solution was purchased from Sigma-Aldrich (2.5 M in
hexane) and was used as received (submitters).
24.
N,N-Dibenzyl-3-methylbutanamide was dried under reduced pressure for 24 h prior to use.
N,N-Dibenzyl-3-methylbutanamide was dissolved in
THF and subsequently transferred to the addition funnel via syringe.
25. ZnBr
2 (98%) was purchased from Alfa Aesar as a white crystalline solid which contained large clumps of reagent. The crystals were lightly ground with a mortar and pestle to provide a finer reagent and subsequently dried using a heat gun (3 x) under reduced pressure (1- 2 mmHg) immediately before use.
26.
NiBr2(dme) (97%) was purchased from Strem and was used as received.
27.
Diethyl allyl phosphate was prepared according to the literature procedure
3e and distilled under vacuum (60 °C at 225 mmHg) before use or used as received from Sigma-Aldrich (checkers).
NiBr2(dme) purchased from other sources resulted in lower yields (submitters).
3g
28. Failure to premix
NiBr2(dme) and
diethyl allyl phosphate or premixing for greater than 1 h resulted in decreased yields.
29. Upon TLC analysis, the R
f value of product
3 in 15%
EtOAc/
hexane was 0.25. The crude reaction mixture was compared to the starting material. The TLC silica gel plate [TLC silica gel 60 F
254, purchased from Merck KGaA (checkers)] was visualized under UV light (254 nm) and then stained with phosphomolybdic acid [FUJIFILM Wako Pure Chemical Corporation (checkers)] (Figure 16).
Figure 16. TLC analysis of the crude product in step C (photo provided by checkers)
30. The column with a 8 cm diameter x 55 cm height was packed with silica gel (350 g) using 7%
EtOAc in
hexane (450 mL). Then, the crude material was loaded onto the column. At that point, fraction collection (300-mL fractions) was begun, and elution was continued with 7.2 L of 7%
EtOAc in
hexane and then 3.3 L of 15%
EtOAc in
hexane. The desired product was obtained in fractions 21-31 (Figure 17).
Figure 17. TLC analysis of column fractions in step B (photo provided by checkers)
31. Product
3 exhibited the following properties: mp = 55 °C; R
f 0.25 (15%
EtOAc in
hexane); IR (cm
-1) 3030, 2915, 1629, 1449, 1229, 1176;
1H NMR
pdf (400 MHz, CDCl
3) δ: 1.82 (s, 3H), 2.03 (s, 3H), 4.45 (s, 2H), 4.59 (s, 2H), 5.93 (s, 1H), 7.16 (d,
J = 7.8 Hz, 2H), 7.23-7.33 (m, 6H), 7.34-7.38 (m, 2H);
13C NMR
pdf (100 MHz, CDCl
3) δ 168.9, 148.4, 137.6, 136.9, 129.0, 128.7, 128.4, 127.7, 127.4, 126.9, 117.8, 50.4, 47.2, 26.6, 20.6; HRMS (ESI)
m/z [M+H]
+ calc'd for C
19H
21NO
+: 280.1696; found 280.1687. Purity was determined to be 98.9% by qNMR
pdf using 20.9 mg of
3 and 12.6 mg of 1,3,5-trimethoxybenzene (FUJIFILM Wako Pure Chemical Corporation, 99.8%) (checkers). When adjusted for purity the yield is 4.74 g (88%). When the reaction was carried out on a half-scale, 2.4 g (88%) of product
3 was obtained.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The α,β-dehydrogenation of carbonyl compounds introduces a synthetic handle that allows for a wide variety of transformations.
2 Recently, the Newhouse group developed a Ni-catalyzed dehydrogenation methodology that uses catalytic
NiBr2(dme) with
diethyl allyl phosphate as oxidant as an expansion on their previously reported palladium-catalyzed dehydrogenation methodology.
3 Development of this nickel-catalyzed dehydrogenation methodology addresses current limitations of allyl-palladium-catalyzed dehydrogenation chemistry. These general conditions have been applied to the dehydrogenation of ketones, esters, nitriles, and amides using LiCyan as base when a ZnBr
2 additive is employed, and can be applied to cyclic ketones, lactones, and lactams when Zn(TMP)
2 is used as base. Importantly, this nickel-catalyzed α,β-dehydrogenation methodology has provided access to substrates previously inaccessible through palladium-catalyzed α,β-dehydrogenation methodology.
The identity of the base used in these reactions had an important effect on the outcome of the reactions. While LiTMP could be used in the Ni-catalyzed dehydrogenation, LiCyan provided the best yields and tolerated a wider variety of substrates. Thus, the development of a new reagent was necessary to access a wide variety of products.
N-Cyclohexyl-2,6-diisopropylaniline, or CyanH, was an adequate precursor for the anilide base, LiCyan. After deprotonation with n-BuLi, LiCyan successfully deprotonated the α-proton of amides and provided high yields of dehydrogenated products using allyl-Ni catalysis. In addition to acting as a base, we believe that Cyan is important in the catalytic reaction itself and may have a role in changing the coordination sphere of the metal.
A selected substrate scope for these reactions is shown in Table 1 and Table 2. In this Org. Synth. article, we describe the nickel-catalyzed α,β-dehydrogenation of an amide using a hindered lithium anilide base. In addition, we detail the preparation of CyanH, the aniline precursor to the anilide base used for the dehydrogenation of these substrates.
Table 1. Representative scope of α,β-dehydrogenated carbonyls using allyl-Ni catalysis and LiCyan
Table 2. Representative scope of α,β-dehydrogenated carbonyls using allyl-Ni catalysis and Zn(TMP)2
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Isovaleryl chloride; (108-12-3)
Dibenzylamine; (103-49-1)
n-Butyllithium; (109-72-8)
Zinc bromide; (7699-45-8)
Nickel (II) bromide dimethoxyethane adduct; (28923-39-9)
2,6-Diisopropylaniline; (24544-04-5)
Cyclohexanone; (108-94-1)
Sodium borohydride; (16940-66-2)
Acetic acid; (64-19-7)
1,2-Dichloroethane; (107-06-2)

|
Alexandra Bodnar was born in Allentown, Pennsylvania and received her B.S. in Biochemistry from the University of Notre Dame in 2019. At Notre Dame, she conducted research under the direction of Professor Brandon Ashfeld, developing transition metal-catalyzed cyclo-additions. Ali is currently a second year Ph.D. student in the Newhouse Group working on developing novel reaction methodologies. She is also interested in the synthesis of complex natural products and the use of computational strategies to access these molecules. |

|
Aneta Turlik was born in Białystok, Poland, and grew up in Brooklyn, NY. In 2013, she received her Bachelor's degree at Barnard College, where she conducted research under the supervision of Professor Christian M. Rojas. She completed her Ph.D. at Yale University in the laboratory of Professor Timothy R. Newhouse (2019). During her time in the Newhouse Group, her research focused on the total synthesis of a diterpene natural product, as well as the development of palladium-catalyzed dehydrogenation reactions. |

|
David Huang was born in New York City and received his B.A. in Chemistry from Princeton University in 2015, working under the supervision of Professor Erik Sorensen in the areas of C-H functionalization and base metal catalysis. He then completed his Ph.D. in 2020 at Yale University with Professor Timothy Newhouse, focusing on the development of palladium and nickel-catalyzed methods for the functionalization of enolates and heteroarenes using unconventional oxidants. |

|
Will Butcher was born outside of Philadelphia, Pennsylvania and received his Bachelor's degree at Bucknell University in 2013. At Bucknell he conducted research under the supervision of Professor Eric Tillman in polymer chemistry. He completed his Master's degree in 2020 at Yale University in the Newhouse Group, where he worked on the total synthesis of complex natural products. |

|
Joanna Lew was born in Durham, North Carolina. She attended North Carolina School of Science and Mathematics before matriculating to Yale as a member of the Class of 2017. During her time at Yale, she performed research in the Newhouse Laboratory. |

|
Tim Newhouse was born in New Hampshire and grew up in northern New England. He received his B.A. in Chemistry from Colby College (2005) in Waterville, ME, where he was mentored by Prof. Dasan M. Thamattoor. After moving to La Jolla, CA, he completed his Ph.D. at The Scripps Research Institute with Prof. Phil S. Baran (2010). During his time at Scripps, he also worked in the laboratories of Prof. Donna G. Blackmond. He then returned to the east coast for postdoctoral studies with Prof. E.J. Corey at Harvard University. He started at Yale in 2013, and is an Associate Professor at Yale University in the Department of Chemistry. |

|
Aoi Takeuchi was born and raised in Kochi, Japan in 1997. He received his Bachelor's degree in Pharmaceutical Sciences from the University of Tokyo in 2020. He continued his graduate studies at the same university under the supervision of Prof. Masayuki Inoue. His studies currently focus on development of synthetic and analytical methodology for structurally complex peptidic natural products. |

|
Hiroaki Itoh was born in Mie, Japan, in 1985. He received his B.Sc. degree in Pharmaceutical Sciences from the University of Tokyo in 2008, and he received his Ph.D. from the same university under the supervision of Prof. Masayuki Inoue. After working for FUJIFILM Corporation for two years, he was appointed as an assistant professor in the Graduate School of Pharmaceutical Sciences at the University of Tokyo. His research interests include the synthesis and chemical biology of biologically active natural products and their analogues with a particular focus on peptidic natural products and related molecules. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved