Org. Synth. 2021, 98, 407-429
DOI: 10.15227/orgsyn.098.0407
Preparation of 6-(Triethylsilyl)cyclohex-1-en-1-yl Trifluoromethanesulfonate as a Precursor to 1,2-Cyclohexadiene
Submitted by Ryo Nakura, Kazuki Inoue, Mayu Itoh, Atsunori Mori, and Kentaro Okano*
1Checked by Zhixun Wang and Kevin Campos
1. Procedure (Note 1)
A. (Cyclohex-1-en-1-yloxy)triethylsilane (2). A 250-mL three-necked round-bottomed flask is equipped with a Teflon-coated magnetic stir bar (3.5 x 1.5 cm), an inlet adapter with a two-way stopcock, and two rubber septa, one of which with a thermocouple inserted. After charged with sodium iodide (9.0 g, 60 mmol, 1.2 equiv) (Notes 2 and 3), the flask is evacuated and backfilled with nitrogen and is charged with anhydrous acetonitrile (80 mL) (Note 4), cyclohexanone (4.9 g, 50 mmol) (Note 5), and triethylamine (8.4 mL, 60 mmol, 1.2 equiv) (Note 6). Triethylsilyl chloride (9.2 mL, 55 mmol, 1.1 equiv) (Note 7) is added to the flask via a syringe through the septum (Figure 1). After stirring at ambient temperature for 3 h (Note 8), the reaction is quenched with water (50 mL).
Figure 1. A) Reaction mixture before the addition of triethylsilyl chloride, B) Reaction mixture during the addition of triethylsilyl chloride, C) Reaction mixture after the addition of triethylsilyl chloride
(photos provided by checkers)
The reaction mixture is transferred into a 500-mL separatory funnel. After partitioning between n-hexane (100 mL) and water twice, the combined organic extracts are washed with sat. aq. NaCl solution (1 x 100 mL), dried over Na2SO4 (25 g), and filtered. The filtrate is concentrated on a rotary evaporator under reduced pressure (15 °C, 10 mmHg), and the residue is dried in vacuo to afford 10.3-11.5 g of a crude product as a colorless oil. The crude product is azeotroped with anhydrous THF (2 x 25 mL) on a rotary evaporator under reduced pressure (40-50 °C, 100 to 20 mmHg) to remove volatiles and water. The resulting crude product (10.84 g) is obtained as a colorless oil in 93% purity (Note 9). The material obtained through this method is used in Step B (Notes 10 and 11) (Figure 2).
Figure 2. (Cyclohex-1-en-1-yloxy)triethylsilane (2) obtained after azeotropic removal with anhydrous THF (photo provided by checkers)
B. 6-(Triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (5). An oven-dried 1-L three-necked flask equipped with two 125-mL pressure-equalizing dropping funnels with a rubber septum, a Teflon-coated magnetic stir bar (4.5 x 1.5 cm), and a rubber septum inserted with a nitrogen gas inlet and a thermocouple is charged with t-BuOK (7.01 g, 62.5 mmol, 2.5 equiv) (Note 12) and anhydrous n-hexane (100 mL) (Note 13). To the suspension is added LDA (1.0 M in THF/hexanes, 62.5 mL, 62.5 mmol, 2.5 equiv) (Note 14) dropwise during 5 min through the pressure-equalizing dropping funnel, and the resulting mixture is stirred at ambient temperature for 30 min (Figure 3).
Figure 3. A) LDA being transferred into the dropping funnel via cannula, B) Reaction mixture after addition of LDA, C) Reaction mixture after addition of (Cyclohex-1-en-1-yloxy)triethylsilane and stirring at ambient temperature for 30 min (photos provided by checkers)
To the reaction mixture is added (Cyclohex-1-en-1-yloxy)triethylsilane (2) (5.73 g of 93% purity, 25.0 mmol) via a syringe during 1 min through the septum (Figure 3). After stirring at ambient temperature for 1.0-2.5 h (Note 15), the reaction mixture is diluted with anhydrous THF (100 mL) (Note 16). After the flask is cooled to -78 °C with a dry ice-acetone bath, Comins' reagent (19.6 g, 50.0 mmol, 2.0 equiv) (Note 17) in anhydrous THF (75 mL) is added to the flask dropwise (Note 18) through the other pressure-equalizing dropping funnel (Figure 4).
Figure 4. A) Reaction mixture before addition of Comins' reagent in THF, B) Reaction mixture after addition of Comins' reagent in THF at -78 ºC, C) Reaction mixture during the warming to ambient temperature
(photos provided by checkers)
The cooling bath is removed after 10 min. After stirring at ambient temperature for 1 h (Note 19), the reaction is quenched with water (100 mL) (Figure 5). The reaction mixture is transferred into a 1-L separatory funnel. After partitioning between n-hexane (150 mL) and water three times, the combined organic extracts are washed with sat. aq. NaCl solution (1 x 150 mL), dried over Na2SO4 (25 g), and filtered. The filtrate is concentrated on a rotary evaporator under reduced pressure (15 °C, 10 mmHg), and the residue is dried in vacuo to afford 12.4-13.5 g of a crude product as a brown oil, which is purified by column chromatography on silica gel while eluting with n-hexane(Note 20) to provide 6-(triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (5) as a colorless oil (7.03 g, 82%) (Note 21) (Figure 6).
Figure 5. Reaction mixture after quench with water (photo provided by checkers)
Figure 6. 6-(Triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (5) (photo provided by checkers)
C. 9,10-Diphenyl-1,2,3,9,9a,10-hexahydro-9,10-epoxyanthracene (8). A 500 mL one-necked round-bottomed flask equipped with a Teflon-coated magnetic stir bar (4.5 x 1.5 cm) is charged with 1,3-diphenylisobenzofuran (6.08 g, 22.5 mmol, 1.5 equiv) (Note 22), 6-(triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (5) (5.5 gof 97% purity, 15.5 mmol, 1 equiv), and THF (165 mL). A thermocouple is inserted into the reaction mixture. Tetrabutylammonium fluoride (1.0 M in THF, 22.5 mL, 22.5 mmol, 1.5 equiv) (Note 23) is added to the flask over a period of 15 min via a syringe (Note 24) (Figure 7), after which the thermocouple is removed.
Figure 7. Reaction mixture after addition of tetrabutylammonium fluoride
(photo provided by submitters)
After stirring at ambient temperature (22-24 °C) for 1.5 h (Note 25), the reaction is quenched with water (150 mL). The reaction mixture is transferred into a 1-L separatory funnel. After partitioning between Et2O (60 mL) and water twice, the combined organic extracts are washed with sat. aq. NaCl solution (1 x 100 mL), dried over Na2SO4 (20 g), and filtered. The filtrate is concentrated on a rotary evaporator under reduced pressure (15 °C, 10 mmHg), and the residue is dried in vacuo to afford 13.9-15.0 g of a crude product as a yellow solid, which is purified by column chromatography on silica gel (Note 26) to provide 9,10-diphenyl-1,2,3,9,9a,10-hexahydro-9,10-epoxyanthracene (8) as a pale yellowish green solid (3.24-3.30 g, 60-61%) (Note 27) (Figure 8).
Figure 8. Concentrated product after flash column chromatography
(photo provided by checkers)
The resulting solid is recrystallized from boiling ethanol (200 mL) (Note 28) in a 500-mL one-necked round-bottomed flask equipped with a reflux condenser and a Teflon-coated magnetic stir bar (4.5 x 1.5 cm). Crystallization is allowed to occur at room temperature over 30 min, then at 0 °C. The crystals are collected by filtration and washed with cold (0 °C) ethanol (20 mL) to provide the title compound 8 (endo/exo >99/1) as a colorless solid (2.43 g, 45%) (Note 29) (Figure 9).
Figure 9. A) Filtration of product (8), and B) appearance of recrystallized product
(photos provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
cyclohexanone,
triethylsilyl chloride,
sodium iodide,
triethylamine,
acetonitrile,
lithium diisopropylamide,
potassium tert-butoxide,
hexane,
tetrahydrofuran,
Comins' reagent,
tetrabutylammonium fluoride,
1,3-diphenylisobenzofuran, as well as the proper procedures for setting up experimental operations.
2.
Sodium iodide (>99.5%) was purchased from Wako Pure Chemical Industries, Ltd. and used pre-dried at 90 °C (bath temperature) with an oil-bath for 18 h under ca. 1 mmHg (Figure 10).
Figure 10. Setup of pre-drying of sodium iodide
(photos provided by submitters)
3. Commercially available anhydrous
sodium iodide (>99.5%), purchased from Sigma-Aldrich Co., LLCby the checkers, works as well as pre-dried material described in
Note 2.
4.
Acetonitrile (>99.8%, water content: < 0.001%) was purchased from FUJIFILM Wako Pure Chemical Corporation and used as received without further purification.
5.
Cyclohexanone (>98.0%) was purchased from Nacalai Tesque Co., Inc. and used as received without further purification.
6.
Triethylamine (>98.0%) was purchased from Nacalai Tesque Co., Inc. and used as received without further purification.
7.
Triethylsilyl chloride (>97.0%) was purchased from Tokyo Kasei Kogyo Co., Inc. and used as received without further purification.
8. The reaction typically requires 3 h to consume all the
cyclohexanone and is monitored by TLC analysis on Merck silica gel 60 F
254 plates developing with
n-hexane/
methyl acetate (9:1). The R
f values of
cyclohexanone (
1) and
(Cyclohex-1-en-1-yloxy)triethylsilane (
2) are 0.16 and 0.68, respectively (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating.
9.
(Cyclohex-1-en-1-yloxy)triethylsilane (
2): colorless oil; R
f = 0.84 (
n-hexane/ethyl acetate = 9:1); Merck silica gel 60 F
254 plates (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating (Figure 11); IR (neat): 2933, 2876, 1667, 1187, 1004, 742, 727 cm
-1;
1H NMR
pdf (500 MHz, CDCl
3) δ: 0.65 (q,
J = 7.9 Hz, 6H), 0.98 (t,
J = 7.9 Hz, 9H), 1.48 - 1.53 (m, 2H), 1.60 - 1.70 (m, 2H), 1.92 - 2.07 (m, 4H), 4.87 (ddd,
J = 3.9, 2.6, 1.3 Hz, 1H);
13C NMR
pdf (126 MHz, CDCl
3) δ: 5.1, 6.7, 22.4, 23.2, 23.9, 29.9, 103.9, 150.4; The purity of the sample was determined to be 93% by
1H qNMR
pdf using 32.7 mg of
1,3,5-trimethoxybenzene (
Note 30) as an internal standard and 58.9 mg of
(Cyclohex-1-en-1-yloxy)triethylsilane (
2), therefore the amount of product in the reaction is 10.08 g. A second reaction on the identical scale provided 11.05 g of the unpurified product with 93% purity.
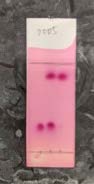
Figure 11. TLC of cyclohexanone (left), reaction mixture (right), and their combination spot (middle). Mobile phase: n-hexane/ethyl acetate = 9:1
(photo provided by checkers)
10. The azeotropic drying method described in Step A was developed as an alternative to column chromatography. The checkers observed that
(Cyclohex-1-en-1-yloxy)triethylsilane (
2) undergoes decomposition on
triethylamine-basified silica gel during flash column chromatography (described in
Note 11), which led to lower yield and purity (79% yield, 85% purity).
11. The submitters reported the chromatographic purification of the crude material. The crude product was dissolved in 0.5% (v/v)
triethylamine in cyclohexane (30 mL) and was charged onto a column (diameter = 10 cm, height = 5 cm) of 95-gram silica gel. The column was eluted with 0.5%
triethylamine in cyclohexane, and 90-mL fractions were collected. Fractions 8-30 were combined and concentrated on a rotary evaporator under reduced pressure (40 °C, 10 mmHg). The submitters used silica gel purchased from FUJIFILM Wako Pure Chemical Corporation (pH = 6.5-7.5, particle size 0.063-0.212 mm), which was used as received.
12.
t-BuOK (>95.0%) was purchased from Tokyo Kasei Kogyo Co., Inc. and used as received without further purification.
13.
hexane (>96.0%, water content: < 30 ppm) was purchased from Nacalai Tesque Co., Inc. and used as received without further purification
14.
LDA (1.0 M in
THF/hexanes) was purchased from Sigma-Aldrich Co., LLC and used as received without further purification. Slightly excess amount (1.5 equiv) of
LDA resulted in incomplete consumption of the starting silyl enol ether.
15. The reaction typically requires 1.0-2.5 h to consume all the
(Cyclohex-1-en-1-yloxy)triethylsilane and is monitored by TLC analysis on Merck silica gel 60 F
254 plates developing with
n-hexane/
methyl acetate (9:1). The R
f values of
(Cyclohex-1-en-1-yloxy)triethylsilane (
2) and 2-(triethylsilyl)cyclohexan-1-one are 0.78 and 0.25, respectively (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating (Figure 12).
Figure 12. TLC of (Cyclohex-1-en-1-yloxy)triethylsilane(left), reaction mixture (right), and their combination spot (middle).
Mobile phase: n-hexane/methyl acetate = 9:1
(photo provided by submitters)
16.
THF (>99.5%, water content: < 10 ppm) was purchased from Kanto Chemical Co., Inc. and further dried by passing through a solvent purification system (Glass Contour) prior to use.
17.
Comins' reagent (>99.0%) was purchased from Oakwood Products, Inc. and used as received without further purification.
18. An exotherm (from -72 to -64 °C) was observed during the 15 min addition time at the reported scale. A larger scale reaction than that described in this procedure may require a longer addition time to control the exotherm.
19. The reaction is monitored by TLC analysis on Merck silica gel 60 F
254 plates developing with
n-hexane/ethyl acetate (9:1). The R
f values of
(Cyclohex-1-en-1-yloxy)triethylsilane and
6-(triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (
5) are 0.90 and 0.50, respectively (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating (Figure 13). The submitters recommend the use of 2-(triethylsilyl)cyclohexan-1-one to monitor the reaction progress.
Figure 13. TLC of (Cyclohex-1-en-1-yloxy)triethylsilane (2, left), cyclohexanone (1, right), reaction mixture containing 5 (middle), the combination of 2 and reaction mixture (2nd from left), the combination of 1 and reaction mixture (2nd from right). Mobile phase: n-hexane/ethyl acetate = 9:1 (photo provided by checkers)
20. The crude product is dissolved in
n-hexane/CH
2Cl
2 = 1:1 (10 mL) and is charged onto a column (diameter = 6.5 cm, height = 9.0 cm) of 59-gram silica gel. The column is eluted with
n-hexane, and 60-mL fractions are collected. Fractions 7-69 are combined and concentrated on a rotary evaporator under reduced pressure (40 °C, 5 mmHg). The submitters used silica gel purchased from FUJIFILM Wako Pure Chemical Corporation (pH = 6.5-7.5, particle size 0.063-0.212 mm), which was used as received. The checkers used silica gel purchased from Fisher Scientific (pH = 4-5, particle size 0.040-0.063 mm), which was used as received. The yield of
5 was not significantly reduced, when acidic silica gel (pH = 4-5) was used as a stationary phase by the checkers.
21.
6-(Triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (
5): colorless oil; R
f = 0.36 (
n-hexane); Merck silica gel 60 F
254 plates (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating; IR (film): 2954, 2878, 1413, 1201, 1141, 1016, 891, 730 cm
-1;
1H NMR
pdf (500 MHz, CDCl
3) δ: 0.64 - 0.70 (m, 6H), 0.97 (t,
J = 8.0 Hz, 9H), 1.43 - 1.54 (m, 1H), 1.62 - 1.73 (m, 2H), 1.90 - 2.01 (m, 1H), 2.02 - 2.15 (m, 2H), 2.15 - 2.27 (m, 1H), 5.65 (ddd,
J = 5.2, 3.3, 1.9 Hz, 1H);
13C NMR
pdf (126 MHz, CDCl
3) δ: 2.9, 7.4, 21.4, 24.2, 25.5, 26.0, 115.1, 118.7 (q,
1JC-F = 321 Hz),153.2;
19F NMR
pdf (471 MHz, CDCl
3) δ: -73.50; The purity of the sample (>97%) was determined by
1H qNMR
pdf using 23.3 mg of
1,3,5-trimethoxybenzene (
Note 30) as an internal standard and 42.7 mg of
6-(triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (
5). Based on the purity of the sample, 6.82 g (79%) of
5 was prepared. A second reaction on the identical scale provided 6.88 g (80%, uncorrected for purity) of the product.
22.
1,3-Diphenylisobenzofuran(>97.0%) was purchased from Tokyo Kasei Kogyo Co., Inc. and used as received without further purification.
23.
Tetrabutylammonium fluoride (1.0 M in
THF) was purchased from Sigma-Aldrich Co., LLC and used as received without further purification.
24. A small exotherm (+5 °C) was observed during 15 min addition time at current scale.
25. The reaction typically requires 1.5 h to consume all the
6-(triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (
5) and is monitored by TLC analysis on Merck silica gel 60 F
254 plates developing with
n-hexane/CH
2Cl
2 (9:1). The R
f values of
6-(triethylsilyl)cyclohex-1-en-1-yl trifluoromethanesulfonate (
5),
endo-
8, and
exo-
8 are 0.60, 0.34, and 0.25, respectively (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating (Figure 14).
Figure 14. TLC of (A) 6-(triethylsilyl)cyclohex-1-en-1-yl trifluoro-methanesulfonate and (B) the reaction mixture
(photo provided by submitters)
26. The crude product is dissolved in CH
2Cl
2 (40 mL) and 30-gram silica gel is added. The resulting slurry is evaporated to dryness on a rotary evaporator (35 °C, 100 to 10 mmHg). The crude product mixture is charged onto a RediSep RF column (diameter = 5.24 cm, height = 18.9 cm, 220-gram silica gel). The column is eluted with linear gradient from 0 to 15% CH
2Cl
2 in
n-hexane on a ISCO CombiFlash system, and 25-mL fractions are collected. Fractions 61-83 are combined (Figure 15) and concentrated on a rotary evaporator under reduced pressure (35 °C, 100 to 10 mmHg). This chromatography method provides product with
endo/
exo ratio of > 99/1.
Figure 15. TLC of the flash column chromatography (fractions 31-86)
(photo provided by checkers)
27. The
1H NMR spectrum of the product shows no impurities; however, the submitters report that elemental analysis of this product did not satisfy purity criterion; therefore, recrystallization was determined to be necessary to provide colorless crystals with appropriate purity.
28. The recrystallization is performed with a Chemglass reaction block as the heat source.
29.
endo-
9,10-Diphenyl-1,2,3,9,9a,10-hexahydro-9,10-epoxyanthracene (
endo-
8): colorless crystals; R
f = 0.34 (
endo) (
n-hexane/CH
2Cl
2 = 9:1); Merck silica gel 60 F
254 plates (stained with an ethanol solution of
p-anisaldehyde). After dipping the TLC plate to the
p-anisaldehyde solution, the chromatogram is stained by heating; mp 128-130 °C (ethanol); IR (film): 3026, 2928, 2844, 1445, 1305, 984, 748 cm
-1;
1H NMR
pdf (500 MHz, CDCl
3) δ: 0.46 (dtd,
J = 13.1, 11.7, 3.2 Hz, 1H), 1.53 - 1.67 (m, 1H), 1.71 - 1.90 (m, 2H), 2.09 - 2.32 (m, 2H), 2.95 - 3.16 (m, 1H), 5.68 (q,
J = 3.2 Hz, 1H), 7.09 - 7.16 (m, 2H), 7.18 - 7.22 (m, 2H), 7.35 - 7.51 (m, 6H), 7.68 - 7.73 (m, 2H), 7.83 - 7.91 (m, 2H);
13C NMR
pdf (126 MHz, CDCl
3) δ: 22.1, 25.0, 26.3, 48.3, 89.9, 90.3, 117.9, 120.3, 121.4, 125.8, 126.9, 127.1, 128.0, 128.3, 128.5, 128.6, 128.7, 135.0, 138.2, 144.4, 144.5, 148.4; The purity of the sample (>98.5%) was determined by
1H qNMR
pdf using 25.8 mg of
1,3,5-trimethoxybenzene (
Note 30) as an internal standard and 21.3 mg of
endo-
9,10-diphenyl-1,2,3,9,9a,10-hexahydro-9,10-epoxyanthracene (
endo-
8). Based on the purity (98.5%) of the sample, 2.39 g (44%) of
endo-
8 was prepared. A second reaction on the identical scale provided 2.51 g (48%, uncorrected for purity) of the product.
30.
1,3,5-Trimethoxybenzene (>99%) was purchased from FUJIFILM Wako Pure Chemical Corporation and used as received.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Among various strained reactive intermediates, 1,2-cyclohexadiene (
6) has attracted considerable attention due to the promising synthetic utility of the bent allene structure (Scheme 1). Since the report of cycloaddition of
1,3-diphenylisobenzofuran with 1,2-cyclohexadiene (
6) by Wittig,
2 a number of papers regarding generation of
6 have been reported.
3 Similar to generation of benzyne from 2-silylphenyl triflate with fluoride ion,
4 6-silyl cyclic enol triflate
9 has been the superior precursor for generating 1,2-cyclohexadiene (
6) so far, because of the mild generation conditions (CsF, rt) and broad functional group compatibilities (Scheme 1).
5 The generated 1,2-cyclohexadiene (
6) undergoes [2+2], [4+2], and [3+2] cycloaddition reactions to provide the corresponding cycloadducts
10-
12.
Scheme 1. Generation of 1,2-cyclohexadiene (6) and its reactions
In spite of the synthetic utility of this compound, the reported method
5a requires multi-step synthesis (Scheme 2). The synthesis commenced with bromination of cyclohexenone (
13) followed by protection of the carbonyl moiety as its acetal
14 to introduce a silyl group via the corresponding alkenyllithium.Acidic aqueous workup provided 2-silylcyclohexenone
15, which was then reduced with L-Selectride and treated the resultant lithium enolate with water to provide α-silylcyclohexanone
16. This
cyclohexanone underwent kinetic deprotonation with
LDA, and the corresponding lithium enolate
17 reacted with PhNTf
2 to givesilylated cyclohexenyl triflate
9, which is the precursor for 1,2-cyclohexadiene (
6).
Scheme 2. First generation synthesis of silylated enol triflate 9
The modified synthesis starts from silylation of the inexpensive
cyclohexanone (
1)to provide silyl enol ether
2(Scheme 3).
6 According to the independent reports from Kuwajima
7 and Corey,
8 a combination of
LDA and
t-BuOK allows for the smooth transfer of the silyl group to the allylic position via the allyllithium, providing the corresponding enolate
3. Subsequent triflation with
Comins' reagent9 provides the precursor
5 for 1,2-cyclohexadiene (
1) in a single flask from silyl enol ether
2 on a 5-gram scale.
Scheme 3. Modified synthesis of silylated enol triflate 5
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Sodium iodide:Sodium iodide; (7681-82-5)
Acetonitrile: Acetonitrile; (75-05-8)
Cyclohexanone: Cyclohexanone; (1) (108-94-1)
Triethylamine: Ethanamine, N,N-diethyl-; (121-44-8)
Triethylsilyl chloride: Silane, chlorotriethyl-; (994-30-9)
t-BuOK: 2-Propanol, 2-methyl-, potassium salt (1:1); (865-47-4)
Hexane: Hexane; (110-54-3)
LDA: 2-Propanamine, N-(1-methylethyl)-, lithium salt (1:1); (4111-54-0)
THF: Furan, tetrahydro-; (109-99-9)
Comins' reagent: Methanesulfonamide, N-(5-chloro-2-pyridinyl)-1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]-; (145100-51-2)
1, 3-Diphenylisobenzofuran: Isobenzofuran, 1,3-diphenyl-; (5471-63-6)
Tetrabutylammonium fluoride: 1-Butanaminium, N,N,N-tributyl-, fluoride (1:1); (429-41-4)
1,3,5-trimethoxybenzene: Benzene, 1,3,5-trimethoxy-; (621-23-8)

|
Ryo Nakura was born in Fukuoka in 1994. He received his B.S. in 2017 and M.S. in 2019 from Kobe University under the supervision of Professors Atsunori Mori and Kentaro Okano. During his M.S. studies, he worked on development of facile preparation of cycloallene intermediates and its application to the synthesis of functionalized cyclohexenes. Currently, he works for Mitsubishi Chemical Corporations. |

|
Kazuki Inoue was born in Osaka in 1994. He received his B.S. in 2017 and M.S. in 2019 from Kobe University under the direction of Professors Atsunori Mori and Kentaro Okano. During his M.S. studies, he worked on generation of strained cycloalkynes and its application to the synthesis of functionalized poly(cycloalkyne)s. Currently, he works for Sumitomo Bakelite Co., Ltd. |

|
Mayu Itoh was born in Kochi in 1996. She received his B.S. in 2019 from Kobe University, where she carried out undergraduate research in the laboratories of Professor Atsunori Mori. In the same year, she then began her graduate studies at the Graduate School of Chemical Science and Engineering, Kobe University under the supervision of under the supervision of Professors Atsunori Mori and Kentaro Okano. Her research focuses on photo-redox reaction of organotin compounds. |

|
Atsunori Mori was born in Aichi in 1959. He received B.S. (1982), M.S. (1984), and Ph.D. (1987) degrees from Nagoya University under the direction of Professor Hisashi Yamamoto. After postdoctoral study in U.C. Berkeley with Peter Vollhardt (1987-1988), he started his academic career as an Assistant Professor at the University of Tokyo (1988) and Japan Advanced Institute of Science and Technology (1993). He was appointed as an Associate Professor in 1995 at Chemical Resources Laboratory of Tokyo Institute of Technology. Since 2005, he has been Professor at the Graduate School of Engineering, Kobe University. |

|
Kentaro Okano was born in Tokyo in 1979. He received his B.S. in 2003 from Kyoto University under the supervision of Professor Tamejiro Hiyama. He then moved to the laboratories of Professor Tohru Fukuyama at the University of Tokyo. In 2007, he joined the faculty at Tohoku University in Professor Hidetoshi Tokuyama's group. In 2014, he visited Professor Amir Hoveyda's laboratories at Boston College as a visiting researcher. In 2015, he moved to Kobe University, where he is currently Associate Professor of Department of Chemical Science and Engineering. His research interest is natural product synthesis based on the development of new synthetic methodologies. |

|
Zhixun Wang joined the Process Research Department of Merck & Co., Inc. in 2019. His research focuses on the synthesis of therapeutic candidates for large-scale production. He received his B. S. degree from Fudan University, and then obtained his Ph.D. under the supervision of Professor Liming Zhang at University of California, Santa Barbara with a research focus on homogeneous gold catalysis. Zhixun then moved to New Haven, CT, where he did postdoctoral work on synthesis of pleuromutilin analogs and structure elucidation of colibactin with Professor Seth Herzon at Yale University. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved