Org. Synth. 2022, 99, 125-138
DOI: 10.15227/orgsyn.099.0125
Ethoxypillar[6]arene
Submitted by Chelsea R. Wilson, Evan F. W. Chen, Austia O. Puckett, and Fraser Hof
1*
Checked by Marc Poirier and Kevin Campos
1. Procedure (Note 1)
Ethoxypillar[6]arene. An oven-dried 2000 mL three-necked round-bottomed flask is equipped with an overhead stirrer (IKA Rw 20 digital) and an argon inlet, the third neck is left unequipped. The vessel is flushed with argon for 10 min (Note 2). 1,4-Diethoxybenzene (40.00 g, 1.0 equiv, 0.24 mol) (Note 3) and paraformaldehyde (14.38 g, 2.0 equiv, 0.48 mol) (Note 4) are charged in the reaction vessel. The solids are agitated at 410 rpm for 5 min under a flow of argon (Figure 1A). Under argon flow, chlorocyclohexane (1.0 L) (Note 5) is added to the reaction vessel through the open inlet. The resulting white cloudy suspension is mixed for 10 min (Figure 1B). The flow of argon is reduced, and a rubber septum is placed on the open neck. While stirring, BF3•Et2O (40 mL, 1.3 equiv, 0.32 mol) (Note 6) is added via syringe (6-inch, 18-gauge stainless steel needle, 50 mL disposal syringe) in a continuous stream (addition time less than 1 min). After approximately 1 min the white cloudy suspension changed to a green suspension with white precipitate (Figure 1C). After approximately 2 min, the suspension changed to a dark green-black suspension (Figure 1D). A small exotherm from 22 °C to 35 °C is observed. The reaction mixture is allowed to cool while stirring for 1.5 h.

Figure 1. A) Reaction vessel charged with paraformaldehyde and diethoxybenzene, B) Suspension of paraformaldehyde and diethoxybenzene in chlorocyclohexane, C) Reaction mixture 1 min after addition of BF3•Et2O, D) Reaction mixture 2 min after addition of BF3•Et2O, E) Reaction mixture immediately prior to quenching, F) Reaction mixture after addition of ethanol, G) Formation of brown precipitate (photos provided by submitters)
After 1.5 h the black chunky suspension (Figure 1E) is quenched by pouring anhydrous ethanol (500 mL) into the reaction vessel (Figure 1F). A brown precipitate forms (Figure 1G) and the suspension is stirred for 5 min (Note 7). The precipitate (Figure 2A) is collected by vacuum filtration using a 20 cm diameter Büchner funnel, a 2 L filter flask, and Whatman 185 mm diameter filter paper (Notes 8 and 9), the precipitate is left to dry under suction for 30 min. The black filtrate (Figure 2B) is discarded and the Büchner funnel is re-equipped with the filter flask. The heterogenous precipitate (Figure 2A) is broken up (Note 10) while under suction. The solid is washed with anhydrous ethanol (300 mL). Once dry, the heterogenous solid is crushed again, breaking up larger pieces. This process is repeated with four more volumes of ethanol (4 x 300 mL) (Note 10). The Büchner flask is emptied before each wash, and each subsequent filtrate becomes clearer and more colorless (Figure 2C) with the solid eventually becoming a fine tan homogenous powder (Figure 2D). After the final wash with ethanol, the solid is left to dry under suction for 1 h.

Figure 2. A) Heterogenous precipitate before ethanol washes, B) Filtrate before ethanol washes, C) Filtrate after fifth ethanol wash, D) homogenous precipitate after fifth ethanol wash (photos provided by submitters)
The dry solid is added to dichloromethane (1000 mL) (Figure 3A), and the suspension stirred at room temperature (820 rpm) for 30 min. The filtrate is collected by suction filtration using a 20 cm diameter Büchner funnel, a 2 L filter flask, and Whatman 185 mm diameter filter paper (Note 11). The precipitate is washed with dichloromethane (3 x 200 mL) (Figure 3B). The organic filtrates are combined (Figure 3C) and concentrated to 400 mL (Note 12) by rotary evaporation (40 °C, 50-200 mmHg) (Figure 3D).
Figure 3. A) Suspension in dichloromethane, B) precipitate from suspension, C) filtrate from suspension, D) filtrate after reducing on rotary evaporator (photos provide by submitters)
The solution is washed with saturated NaHCO3 (3 x 300 mL) (80 g in 1 L distilled H2O) followed by a brine wash. The brown solution (Figure 4A) turns yellow after the brine wash (Figure 4B) (Note 13). The combined aqueous layers are washed with dichloromethane (100 mL). The organic layers are combined (Figure 4C) and dried over MgSO4 (20 g). The drying agent is collected with suction filtration using an 8.5 cm diameter Büchner funnel, 1 L filter flask, and Whatman 70 mm diameter filter paper, and this solid is washed with dichloromethane (2 x 50 mL). The filtrate is concentrated by rotary evaporation (40 °C and gradually increased to 80 °C, 50-200 mmHg). The solid is further dried under vacuum (0.3 mmHg) with heating (50 °C) using an oil bath for 48 h (Note 14). Ethoxypillar[6]arene (19.40 g) is obtained as a white-yellow powder (Figure 4D, 84% purity, 45% yield, 38% corrected for purity) (Note 15).
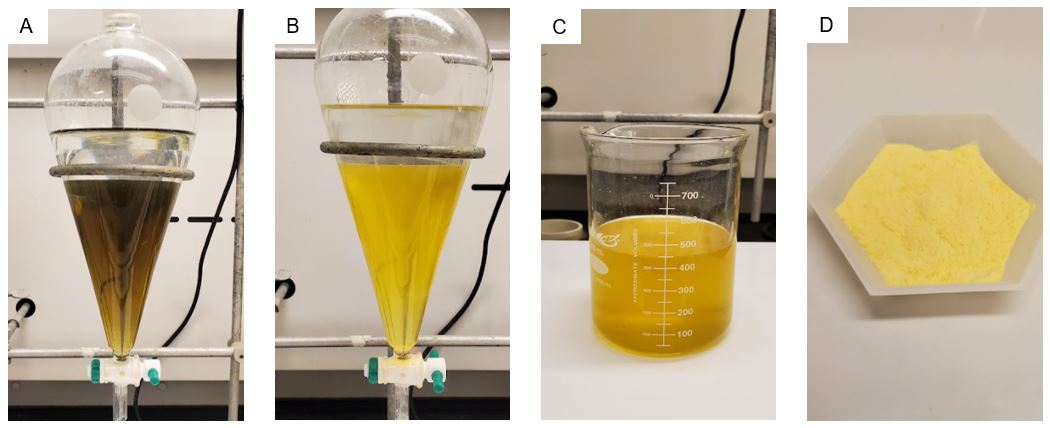
Figure 4. A) Filtrate after one wash with brine, B) Filtrate after three brine washes, C) Combined organic layers, D) EtOP6 (photos provided by submitters)
The crude ethoxypillar[6]arene is recrystallized (Note 16) by dissolving the crude material in chloroform (600 mL) in a 2 L round-bottomed flask, and acetone (600 mL) is then added to the solution (Figure 5A). The round-bottomed flask is then placed in a -20 °C freezer for 24 h. The precipitate (Figure 5B) is collected by suction filtration using a 20 cm diameter Büchner funnel, a 2 L filter flask, and Whatman 185 mm diameter filter paper. The solid is further dried under vacuum (55 °C, 0.3 mmHg) for 48 h (Note 17). Ethoxypillar[6]arene (13.32 g) is collected as a white crystalline solid (Figure 5C, 97% purity) (Note 19). The filtrate is placed in the -20 °C freezer for an additional 24 h, and an additional portion of ethoxypillar[6]arene (1.06 g) is obtained after drying in the same manner as above (Notes 18 and 19).
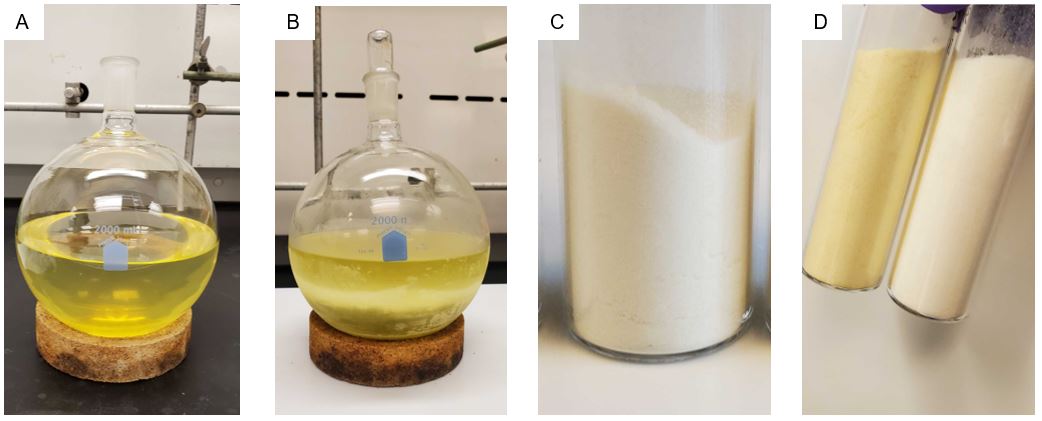
Figure 5. A) ethoxypillar[6]arene dissolved in chloroform and acetone, B) Ethoxypillar[6]arene crystallization, C) Recrystallized ethoxypillar[6]-arene, D) crude ethoxypillar[6]arene (left) and recrystallized Ethoxypillar[6]arene (right) (photos provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
1,4-diethoxybenzene,
paraformaldehyde,
chlorocyclohexane,
boron trifluoride ethyl etherate,
ethanol,
dichloromethane,
sodium carbonate,
magnesium sulfate,
ethoxypillar[6]arene,
chloroform,
2,4-dinitrochlorobenzene, and
acetone.
2. The checker used nitrogen as inert gas delivered through a needle in a septum. A temperature probe was inserted through the septum and used to monitor the reaction temperature.
3.
1,4-Diethoxybenzene (>98.0%) was obtained from TCI and was used as received. The checker used
1,4-diethoxybenzene obtained from Oakwood Chemical (98%), and the solid was ground in a mortar and pestle prior to use.
4.
Paraformaldehyde (95.0%) was obtained from Sigma-Aldrich and was used as received.
5.
Chlorocyclohexane (>98.0 %, synthesis grade) was obtained from Sigma-Aldrich and was used as received.
6.
Boron trifluoride ethyl etherate (synthesis grade) was obtained from Sigma-Aldrich and was used as received.
7. The reaction mixture should be stirred until all the black suspension has been quenched and turns to a brown precipitate. A spatula may be required to break up large chunks of solids.
8. An additional 200 mL of
ethanol (anhydrous) was used to rinse out the reaction vessel.
9. A large diameter funnel is recommended to speed up the filtration process. The checkers used a 2L medium porosity fritted filter funnel (Chemglass, 132mm).
10. The bottom of a clear glass crystallization dish works well to crush the heterogenous solid. When the solid is dry, crush/grind with the dish. The more homogenous the precipitate the more
chlorocyclohexane is removed and the more product can be obtained from the black solid masses. Additionally, the subsequent filtration will be quicker with a finer precipitate. Note that this process will have no effect on the ring-size selectivity of the reaction, only the yield.
11. It is important that the filtrate does not run dry under suction. On smaller scale reactions it might be required to add additional
dichloromethane to the Büchner funnel. The checker used a 2L medium porosity fritted filter funnel (Chemglass, 132mm) and added celite-545 (0.5wt%, 20g) as filter-aid.
12. Caution should be used when concentrating by rotary evaporation - do not remove all the solvent! Removing too much solvent can cause the compound to degrade and form an insoluble polymer. The volume should not be reduced below 400 mL. If a brown film-like precipitate forms, immediately remove the solution from the rotary evaporator, add more
dichloromethane and filter off the insoluble precipitate. Continue the protocol with the remaining filtrate. Note on smaller scale reactions this step can be omitted, this step is to reduce the volume for easier management of large-scale reactions.
13. An additional brine wash might be required if the filtrate has not turned yellow. The checkers measured the pH of the aqueous phase after the 3
rd NaHCO3 wash to ensure pH≥9 .
14. This additional drying step is to remove residual
dichloromethane that is trapped inside the cavities in the crystalline solid. This step may be omitted based on the level of purity required. The checker used a vacuum oven at 50 °C and 680 mmHg for drying the solid.
15. Purity of crude
ethoxypillar[6]arene was determined to be 84% by quantitative
1H NMR
pdf with
2,4-dinitrochlorobenzene (
Note 20) as the internal standard. A mixture of 6.21 mg of
ethoxypillar[6]arene and 5.7 mg of
2,4-dinitrochlorobenzene was used to determine purity. Crude
ethoxypillar[6]arene is a yellow-tan solid that is bench stable at room temperature in air.
16. Recrystallization removes residual
chlorocyclohexane, this step may be omitted based on the level of purity required.
17. This additional drying step is to remove residual
acetone that is trapped inside cavities in the crystalline solid. This step can be omitted based on the level of purity required.
18. Characterization of
ethoxypillar[6]arene:
1H NMR
pdf (500 MHz, CDCl
3) d: 6.70 (s, 12 H), 3.81 (q,
J = 7.0 Hz, 24 H) overlapping 3.80 (s, 12 H), 1.30 (t,
J = 7.0 Hz, 36 H);
13C NMR
pdf (126 MHz, CDCl
3) d: 150.5, 127.9, 115.3, 64.0, 31.0, 15.2; HR-MS (ESI)
m/z calcd for C
66H
85O
12+ 1069.6041 [
M+H]
+; found 1069.6016. mp 165-167 °C. Purity of recrystallized
ethoxypillar[6]arene was determined to be 98.2% by quantitative
1H-NMR
pdf with
2,4-dinitrochlorobenzene (
Note 20) as the internal standard. A mixture of 5.4 mg of
ethoxypillar[6]arene and 6.4 mg of
2,4-dinitrochlorobenzene was used to determine purity.
19. A second reaction performed by the checkers on half-scale provided 7.9g (36%) of a white crystalline solid with 98% purity.
20.
2,4-Dinitrochlorobenzene (97%) was obtained from Sigma-Aldrich and was used as received.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Pillar[n]arenes are a family of macrocyclic hosts first reported in 2008
2 that have quickly become an important scaffold in supramolecular chemistry. This pillar-shaped (cylindrical) macrocycle consists of 'n' repeating hydroquinone units linked through 'n' methylene bridges. Pillar[5]arene was the first pillar[n]arene synthesized, and remains dominant in the field due to the ease of synthetic access to this macrocycle size.
3 Pillar[5]arene is the least strained pillar[n]arene and is the dominant product under most synthetic conditions.
4, 5, 6First made in 2009,
6 pillar[6]arene has become a highly desired analog due to its ability to bind larger, biologically relevant guest molecules.
7 Synthesis of pillar[6]arene is challenging due to the competing formation of pillar[5]arene and accompanying purification problems. The formation of alkoxypillar[6]arene is disfavored due to the steric strain and distorted bond angles of the pillar[6]arene macrocycle. While methods to make alkoxypillar[6]arenes have been reported, they all involve challenging chromatographic separations of pillar[5]arene byproducts that cause problems with reproducibility, limit the purity of pillar[6]arene product, and limit the ability to work at the larger scales (see below).
We have developed a method that makes ethoxypillar[6]arene, which is a key intermediate that can be used in the synthesis of almost all elaborated pillar[6]arenes. Our method generates pure 6-membered macrocycle with scant 5-membered macrocycle byproducts, and the product is easily purified without the need for column chromatography. It is easily scalable from 1.0 g up to 40 g batches, making it an attractive method for large scale synthesis.
While there are reported methods to make
ethoxypillar[6]arene4,5,6, 8,9,10,11,12,13,14,15,16,17,18 they have significant drawbacks. They are not ring-size selective and/or are not easily scaled up. A method using [choline chloride][FeCl
3]
2 as a "deep-eutectic solvent" (53% yield)
8 and a method that uses polar solvents to template the cyclization (72% yield) have been reported.
15 However, these methods require chromatography and were only performed on a ≤1.66 g scale. An FeCl
3-catalyzed cyclization resulted in a 34% yield of
ethoxypillar[6]arene on a 500 mg scale, however this was also unselective.
5 A solvent-free mechanochemical method suggests a selective synthesis of
ethoxypillar[6]arene on a 1.66 g scale with an 84% yield.
14 This method was published in 2016 and has not become common in the pillar[6]arene literature. Our method solves the issues the previous methods have with scalability and selectivity.
Our method combines aspects of previously reported methods.
Chlorocyclohexane was reported to template the synthesis of a dodeca-cyclohexylmethyl-pillar[6]arene.
18 While this resulted in a high yield of 87%, it was not ring-size selective and the bulky cyclohexylmethyl protecting group is very hard to remove and limits the ability for elaborate functionalization.
BF3•Et2O is a common Lewis acid catalyst used in the cyclization of the pillar[n]arene macrocycles.
10,11,12, 15,16,17,18 By combining the templating effect of
chlorocyclohexane with the common Lewis acid catalyst we are able to achieve a ring-size selective method that requires no chromatography. This method will enable researchers to work on larger scales and explore more potential applications in both medicine and materials science.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
1,4-Diethoxybenzene: Benzene, 1,4-diethoxy-; (122-95-2)
Paraformaldehyde: Paraformaldehyde; (30525-89-4)
Chlorocyclohexane: Cyclohexane, chloro-; (542-18-7)
BF3•Et2O: Boron, trifluoro[1,1'-oxybis[ethane]]-, (T-4); (109-63-7)
Ethanol: Ethanol; (64-17-5)
Dichloromethane: methane, dichloro-; (75-09-2)
NaHCO3: Carbonic acid sodium salt (1:1); (144-55-8)
MgSO4: Magnesium sulfate anhydrous, (7487-88-9)
Ethoxypillar[6]arene:Heptacyclo[26.2.2.23,6.28,11.213,6.218,21.223,26]dotetraconta-3,5,8,10,13,15,18,20,23,25,28,30,31,33,35,37,39,41-octadecaene, 4,9,14,19,24,29,31,33,35,37,39,41-dodecaethoxy-; (1207685-12-8)
Chloroform: Methane, trichloro-; (67-66-3)
Acetone: 2-Propanone; (67-64-1)
2,4-Dinitrochlorobenzene: Benzene, 1-chloro-2,4-dinitro-; (97-00-7)

|
Chelsea Wilson was born in Dartmouth, Nova Scotia and raised in North Saanich, British Columbia. She earned her B.Sc. (Hons.) in Chemistry at the University of Victoria. During her undergraduate degree she conducted research with Dr. Katherine Elvira, Dr. David Berg, Dr. Peter Wan and Dr. Fraser Hof. The fields of research covered microfluidics, inorganic and organic synthesis, photochemistry and medicinal chemistry. In 2019 she started her Ph.D. at the University of Victoria with Dr. Fraser Hof. Chelsea has a passion for supramolecular and medicinal chemistry. |

|
Evan Chen received his B.Sc. in Chemistry with distinction from the University of Victoria. During his time at the University of Victoria, he partook in research projects with Dr. Fraser Hof on macrocycle synthesis and on medicinal chemistry. Afterwards, he decided to pursue graduate studies in organic synthesis and catalysis at McGill University under the supervision of Dr. Chao-Jun Li. He is currently working on a project in green chemistry on the development and applications of methodology for cross-coupling reactions involving hydrazone substrates. |

|
Austia Puckett is an undergraduate student working towards her B.Sc. (Honours) in Chemistry at the University of Victoria. She is interested in organic and medicinal chemistry and is currently working on an Honours thesis research project under the guidance of Dr. Fraser Hof and Chelsea Wilson on the subject of pillar[6]arenes for biomedical applications. |

|
Fraser Hof was born and raised in Medicine Hat, Canada. He earned his B.Sc. at the University of Alberta, doing research with David Bundle, Derrick Clive, and Neil Branda, as well as an internship in a process scale-up lab under Frank Weisner at Raylo Chemicals (now Gilead Sciences). In 1998 he earned a Ph.D. in supramolecular chemistry at The Scripps Research Institute, La Jolla, with Julius Rebek, Jr. He was then a post-doctoral fellow studying medicinal chemistry with François Diederich at ETH Zürich. He started at the University of Victoria in 2005, where he is now Professor of Chemistry. Fraser is fascinated by water and is motivated by the potential of host-guest systems to solve important problems in biomedical research. |

|
Marc Poirier was born in New Brunswick, Canada. He obtained his graduate degree in organic chemistry (MS; Organic chemistry) in 1995 at the Université de Moncton, N.B., Canada. He is currently working at Merck Research Laboratories in Rahway, NJ with over 25 years of experience in Process Chemistry and Discovery Process Chemistry. His research interests span from traditional process chemistry development to flow chemistry and biocatalytic transformations. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved