Org. Synth. 2022, 99, 159-173
DOI: 10.15227/orgsyn.099.0159
Synthesis of Phenanthridinones via the Palladium-Catalyzed Annulation of Benzyne
Submitted by Dominick C. Witkowski, Luca McDermott, and Neil K. Garg*
1Checked by Russell D. Cink and Seble Wagaw
1. Procedure (Note 1)
5-Ethylphenanthridin-6(5H)-one (3). A single-necked (24/40 joint) 1000 mL round-bottomed flask is equipped with a Teflon-coated magnetic stir bar (4.0 x 1.5 cm, football-shaped). The flask is charged with palladium(II) acetate (221 mg, 984 µmol, 5 mol%) (Note 2), bis(diphenylphosphino)methane (758 mg, 1.97 mmol, 10 mol%) (Note 3), sodium carbonate (2.09 g, 19.7 mmol, 1 equiv) (Note 4), 2-bromo-N-ethylbenzamide (1) (4.50 g, 19.7 mmol, 1 equiv) (Note 5), and cesium fluoride (15.0 g, 98.7 mmol, 5 equiv) (Note 6), each in one portion. The flask is sealed with a 24/40 rubber septum with a nitrogen inlet and an outlet connected to a bubbler, and the flask is then flushed with nitrogen for 5 min. Dry toluene (308 mL) (Note 7) and dry acetonitrile (77.0 mL) (Note 8) are added sequentially to the flask via cannula to give a suspension. 2-(Trimethylsilyl)phenyl trifluoromethanesulfonate (2) (9.58 mL, 11.8 g, 39.5 mmol, 2 equiv) (Note 9) is then added over 2 min using a plastic syringe. The reaction flask is purged with nitrogen for 5 min and allowed to stir (750 rpm) at 23 ºC. During this time, a jacketed reflux condenser (3.5 cm OD x 25 cm tall, 24/40 joint) is capped with a rubber septum and flushed with nitrogen through an 18G x 1.5" needle attached to a nitrogen line. After 5 min the reaction flask is fitted with the condenser. The outside of the joint between the condenser and the 1000 mL round-bottomed flask is wrapped tightly with Teflon tape and clamped with a 24/40 keck clip. The apparatus is purged with nitrogen for 5 min and then cold water is flowed through the condenser. The flask is then placed in an oil bath preheated to 110 ºC and allowed to stir (750 rpm) for 24 h while under positive pressure of nitrogen (Figures 1 and 2).

Figure 1. Reaction setup (photo provided by submitters)
Figure 2. Reaction mixture at A) 0 min, B) 30 min, C) 24 h after the reaction mixture is placed in the oil bath (photos provided by submitters)
After 24 h (Note 10), the flask is removed from the oil bath and allowed to cool to 23 °C over a time period of 30 min while stirring. The flask is then opened to the air, and the stir bar is removed. The contents of the flask are transferred into a 3000 mL separatory funnel. Saturated sodium chloride solution (200 mL) (Note 11) and ethyl acetate (200 mL) (Note 12) are then added to the flask and transferred to the separatory funnel. The funnel is shaken, and the layers are separated (Note 13). The aqueous layer is then extracted with ethyl acetate (2 x 200 mL) (Note 14).
Figure 3. A) Reaction mixture after transferring to separatory funnel. B) Mixture after first extraction. C) Mixture after second extraction (photos provided by submitters)
The organic layers are combined, dried over magnesium sulfate (15 g) (Note 15), then vacuum filtered through a 150 mL medium porosity fritted Büchner funnel into a 2000 mL round-bottomed flask. The filtrate is concentrated by rotary evaporation under reduced pressure (30 °C to 35 °C, 175 mmHg to 20 mmHg) to afford an orange solid.
The 2000 mL round-bottomed flask with the crude product is charged with silica gel (13.0 g) (Note 16). The crude material and silica gel are suspended in methylene chloride (75 mL) (Note 17) and concentrated under reduced pressure (30 °C, 350 mmHg to 175 mmHg). The product-adsorbed silica is then dried on high vacuum (<1.0 mmHg) for 1 h until fine and powdery (Note 18). The adsorption process is repeated with silica gel (2 x 1 g) and methylene chloride (2 x 25 mL) to facilitate quantitative recovery (Note 19).
The product-adsorbed silica is then charged on a column (8 cm OD x 14 cm tall) that is wetted using 288 g of silica gel and 1000 mL 9:1 hexanes:ethyl acetate (Notes 12, 16, 20) (Figure 4).
Figure 4. Column dry-loaded with product-adsorbed silica (photo provided by submitters)
The column is eluted with 9:1 hexanes:ethyl acetate (9400 mL) and collected into 55 mL culture tubes. The desired product elutes in fractions 77-164 (Note 21). These fractions are pooled and concentrated under reduced pressure (30 °C, 70 mmHg). The resulting orange oil is collected in an 8-dram vial which is subsequently equipped with a vacuum adapter and dried under high vacuum at 23 °C (<1.0 mmHg for 1 h) to afford 5-ethylphenanthridin-6(5H)-one (3) as an orange solid (2.47 g, 56%) (Figure 5) (Notes 22, 23, and 24).
Figure 5. Isolated 5-ethylphenanthridin-6(5H)-one (3) (photo provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
palladium(II) acetate,
bis(diphenylphosphino)methane,
sodium carbonate,
2-bromo-N-ethylbenzamide,
2-(trimethylsilyl)phenyl trifluoromethanesulfonate,
cesium fluoride,
toluene,
acetonitrile,
ethyl acetate,
hexanes,
methylene chloride,
magnesium sulfate and silica gel.
2.
Palladium(II) acetate (>99%) was purchased from Strem Chemicals, Inc. and used as received.
3.
Bis(diphenylphosphino)methane (97%) was purchased from Sigma-Aldrich Corp. and used as received.
4.
Sodium carbonate powder was purchased from EMD Millipore and used as received. If granular
sodium carbonate is used it should be ground to a fine powder with a mortar and pestle immediately prior to use.
5.
2-Bromo-N-ethylbenzamide (
1) (98%) was purchased from Combi-Blocks and used as received.
6.
Cesium fluoride (99+%) was purchased from Strem Chemicals, Inc. and used as received.
7.
Toluene (≥ 99.5%) was purchased from Fisher Scientific. It was passed through an activated alumina column before use.
8.
Acetonitrile (99.7%) was purchased from Fisher Scientific. It was passed through an activated alumina column before use.
9.
2-(Trimethylsilyl)phenyl trifluoromethanesulfonate (
2) (96%) was purchased from Combi-Blocks and used as received.
10. The progress of the reaction is monitored by TLC analysis on silica gel with 5:1
hexanes:
EtOAc used as an eluent. The plate is visualized using a UV lamp (254 nm). The
2-bromo-N-ethylbenzamide starting material has R
f 0.07 and the
5-ethylphenanthridin-6(5H)-one (
3) product has R
f 0.31 (Figure 6).
Figure 6. TLC of the crude reaction mixture after 24 h (S = benzamide starting material, C = co-spot of S and R, and R = reaction mixture) (photo provided by submitters)
11.
NaCl (>99.5%) was purchased from VWR and used as received.
12.
Ethyl acetate (99.5%) was purchased from VWR and used as received.
13. A black emulsion forms at the interface of the organic and aqueous layers. The emulsion is collected with the aqueous layer.
14. During the second extraction, the black emulsion is collected with the aqueous later. In the third extraction, the black emulsion is collected with the organic layer.
15.
Magnesium sulfate (≥99%) was purchase from J. T. Baker and used as received.
16. SiliaFlask P60 (particle size 0.040-0.063 nm) was purchased from SiliCycle and used as received.
17.
Methylene chloride (99.9%) was purchased from Fisher Scientific and used as received.
18. A Kim-wipe is packed into the opening of the vacuum adapter to avoid loss of silica gel (Figure 7).
Figure 7. The product-adsorbed silica placed under high-vacuum with a Kim-wipe packed in the opening of the vacuum adapter (photo provided by submitters)
19. The additional treatments with silica were found to be helpful in ensuring complete removal of the silica-adsorbed material from the flask.
20.
Hexanes (>98.5%) was purchased from Fisher Scientific and used as received.
21. Fractions containing the product were identified by TLC analysis (using 9:1
hexanes:
EtOAc as eluent, where
3 has an R
f 0.20. Fractions 64-76 contained desired product and one impurity (R
f 0.23). These fractions were not collected. Fractions 77-164 contained the desired product, and each fraction is rinsed with (2 x 1 mL)
EtOAc to ensure quantitative transfer. Fractions 165-188 contained desired product and one impurity (R
f 0.10). These fractions were not collected. (Figure 8).
Figure 8. Representative TLC analysis showing impurities in column fractions (photo provided by submitters)
22. The product can be characterized as follows: IR (Ge IRE): 3073, 2970, 1649, 1636, 1608, 1462, 1456, 1435, 1366, 1355, 1342, 1314, 1109, 753, 744, 726, 689 cm
-1; mp 87-90 °C;
1H NMR
pdf (400 MHz, CDCl
3) δ: 8.55 (dd,
J = 8.0, 1.5 Hz, 1H), 8.31 - 8.22 (m, 2H), 7.74 (ddd,
J = 8.4, 7.1, 1.4 Hz, 1H), 7.61 - 7.49 (m, 2H), 7.42 (d,
J = 8.9 Hz, 1H), 7.29 (ddd,
J = 8.2, 7.1, 1.2 Hz, 1H), 4.46 (q,
J = 7.1 Hz, 2H), 1.42 (t,
J = 7.1 Hz, 3H);
13C NMR
pdf (101 MHz, CDCl
3) δ: 161.2, 137.0, 133.6, 132.5, 129.7, 128.9, 128.0, 125.7, 123.6, 122.4, 121.7, 119.6, 115.0, 37.8, 12.8; HRMS-ESI (
m/z) [M + H]
+ calcd for C
15H
14NO, 224.1070; found, 224.1069.
23. The purity of
3 was determined to be >97 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Sigma-Aldrich, >99.9%) as the internal standard.
24. A second reaction performed by the checkers at the identical scale provided 2.70 g (61%) of the product.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure described provides an efficient synthesis of substituted phenanthridinones through the transition metal-catalyzed annulation of benzyne. Though strained cyclic intermediates, such as arynes and cyclic alkynes, were initially considered mere scientific curiosities, pioneering experiments by Roberts
2,3 and Wittig
4 in the 1950s validated the existence of benzyne and prompted further study of these intermediates. Their high degree of ring strain gives rise to unique and synthetically useful reactivity. In recent decades, aryne chemistry has undergone a substantial period of growth, leading to their use as building blocks in the synthesis of heterocycles,
5 ligands,
6 natural products,
7,8,9 agrochemicals,
10 and organic materials
11 (Figure 9). Moreover, arynes can be generated through the mild and safe fluoride-induced 1,2-elimination of
o-(trimethylsilyl)aryl triflates, and these precursors are now extensively utilized.
12,13,14 Many modes of aryne reactivity have been explored with the three most common reaction classes being nucleophilic additions, cycloadditions, and transition metal-catalyzed reactions (used to access compounds
4,
5, and
6 respectively). This procedure describes a transition metal-catalyzed annulation reaction to access phenanthridinones.
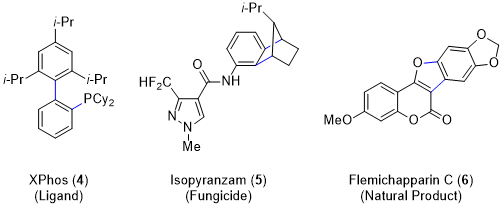
Figure 9. Select synthetic applications of arynes
As shown in the selected scope (Figure 10), the palladium-catalyzed annulation is tolerant of variation of the
o-halobenzamide component. Specifically, phenanthridinones with both electron-donating (
16a and
16c) or electron-withdrawing substituents (
16b and
16e), as well as halogens (
16d), could be formed in good yields from the corresponding
o-halobenzamides. Additionally, heterocycles could be incorporated into the product scaffold as demonstrated by the formation of aza-phenanthridinone
16f. The methodology also proved tolerant of variation of the
N-substituent, enabling the formation of
N-allyl and
N-
tBu phenanthridinones
16g and
16h, respectively. Notably, this annulation generates privileged phenanthridinone scaffolds, which can be found in natural products and pharmaceuticals. Numerous methods for their syntheses have been described, many involving multiple steps.
15 The method described stands in contrast to previous aryne-based approaches to the phenanthridinone scaffold, which require harsher reaction conditions.
16
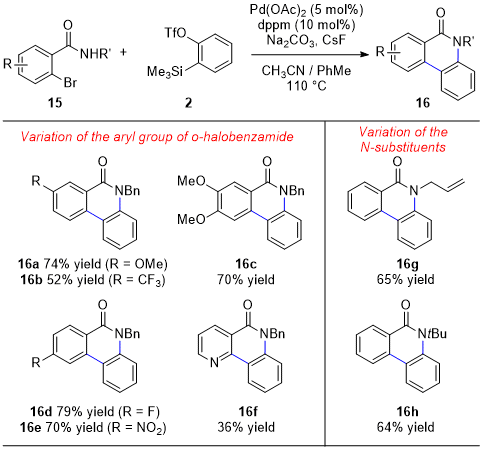
Figure 10. Selected scope of phenanthridinones accessible with various o-halobenzamide derivatives
Beyond the synthesis of phenanthridinones,
17 palladium-catalyzed aryne annulations have proven to be quite general, enabling access to diverse product scaffolds from appropriately substituted precursors bearing an aryl halide and a pendent nucleophile or electrophile (e.g.,
7,
9,
11,
13, Figure 11). For example, 9-fluorenylidene (
8) and fused polycyclic aromatic (
12) are accessible via palladium-catalyzed annulation of arynes with
o-halostyrenes
18 and aryl halides,
19 respectively. 9
H-Fluroen-9-one (
10) can be synthesized via a palladium-catalyzed annulation of arynes with
o-iodobenzaldehyde (
9).
20 And, protected
o-iodoanilines (e.g.
13) were successful in delivering carbazole
14.
21This reaction design has also proven useful for in situ-generated cyclic allenes. Reactions of strained cyclic allenes are relatively less developed than those of related classes of fleeting intermediates
22 despite cyclic allenes having distinct advantages for the synthesis of sp
3-rich and stereochemically complex targets. The annulation of 1,2-cyclohexadiene, resulting from silyl triflate
16, with
o-halonucleophiles, such as
o-iodobenzamide
15, proceeds via the formation of two new bonds and a sp
3 center (Figure 11). The palladium-catalyzed annulation of 1,2-cyclohexadiene and related derivatives with
o-halo nucleophiles represents only the third known example of a metal-catalyzed reaction of strained cyclic allenes in the literature.
23,24,25Figure 11. Overview of annulations of strained intermediates with o-halo nucleophiles
Overall, this procedure provides a simple approach to access phenanthridinones from silyl triflates and o-halobenzamides. The ready availability of the starting materials and its tolerance toward various aryl substituents, N-substituents, and functional groups makes it an attractive strategy. Furthermore, this strategy for intercepting arynes with transition metal-catalysis has also proven applicable to other strained intermediates such as cyclic allenes. These efforts hopefully will engender further research into transition metal-catalyzed reactions of transient strained intermediates for the synthesis of complex scaffolds.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
2-Bromo-N-ethylbenzamide; (1) (80031-02-3)
2-(trimethylsilyl)phenyl trifluoromethanesulfonate; (2) (88284-48-4)
Palladium(II) acetate; (3375-31-3)
Bis(diphenylphosphino)methane; (2071-20-7)
Cesium fluoride; (13400-13-0)
Sodium carbonate (497-19-8)

|
Dominick Witkowski was born in Portsmouth, VA and raised in Northborough, MA. In 2020, he received his B.A. in Chemistry from Boston University where he carried out research under the direction of Professor John A. Porco, Jr. He then moved to the University of California, Los Angeles where he is currently a second-year graduate student in Professor Neil K. Garg's laboratory. His studies primarily focus on developing synthetic methods utilizing strained cyclic allenes. |

|
Luca McDermott was born and raised in San Francisco, CA. In 2020, he received his B.S. in Biochemistry from Tufts University where he carried out research under the direction of Professor Clay S. Bennett. He then moved to the University of California, Los Angeles where he is currently a second-year graduate student in Professor Neil K. Garg's laboratory. |

|
Neil Garg is a Distinguished Professor of Chemistry and the Kenneth N. Trueblood Endowed Chair at the University of California, Los Angeles. His laboratory develops novel synthetic strategies and methodologies to enable the total synthesis of complex bioactive molecules. |

|
Russell D. Cink graduated from the University of Minnesota in 1991 with a B.S. in Chemical Engineering. While an undergraduate, he conducted research under the direction of Wayland E. Noland which sparked his interest in synthetic organic chemistry. After working for 2 years as a consultant, he returned to the University of Minnesota and obtained a Ph.D. in Chemistry in 1998 under the direction of Craig J. Forsyth. Since 1998 he has worked as a Process Chemist at Abbott / AbbVie, primarily focused on small molecule process development and recently antibody drug conjugates. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved