Org. Synth. 2022, 99, 326-341
DOI: 10.15227/orgsyn.099.0326
Stereoselective Synthesis of Dimethyl 4(S)-Allyl-N-Boc-L-glutamate and Related Congeners
Submitted by Akash Mishra and Stephen Hanessian*
1Checked by Qin Han Teo and Pauline Chiu
1. Procedure (Note 1)
Dimethyl (2S,4S)-2-allyl-4-((tert-butoxycarbonyl)amino)pentanedioate (2). An oven-dried, 250-mL, three-necked, round-bottomed flask (14/23, 19/26, 14/23 ground glass joints) equipped with a Teflon-coated magnetic stir bar (3.5 × 1.0 cm, egg-shaped), is charged with (S)-dimethyl 2-((tert-butoxycarbonyl)amino)pentanedioate (1) (3.00 g, 10.9 mmol, 1.00 equiv) (Notes 2 and 3). The flask is fitted with a vacuum adapter (14/23 ground glass joint) that is connected to a double manifold, while the other two necks are stoppered with glass stoppers (19/26 and 14/23 ground glass joints) (Figure 1A). The flask is evacuated (< 1 mmHg) and refilled with argon three times. Under a flow of argon, the center 19/26 glass stopper is replaced by a rubber septum, which is pierced with a 21G needle connected to the argon line, and an 18G needle connected to an oil bubbler. The other 14/23 glass stopper is replaced under argon by a glass thermometer adapter (14/23 ground glass joint), fitted with a low temperature thermometer (Figure 1B). Anhydrous tetrahydrofuran (45 mL, 0.24 M) (Note 4) is added in one portion at room temperature via a plastic syringe fitted with a 18G needle through the rubber septum under argon. The flask is cooled to -78±1 °C using a dry ice/acetone bath in a Dewar flask (Figure 1C) (Note 5). After 10 min, a solution of lithium bis(trimethylsilyl)amide 1.0 M in THF (24 mL, 24 mmol, 2.2 equiv) (Note 6) is added dropwise via a plastic syringe fitted with a 18G needle over the course of 25 min under a positive flow of argon. The rate of the addition of LiHMDS solution was such that the internal temperature of the reaction mixture is maintained at -78±1 °C.
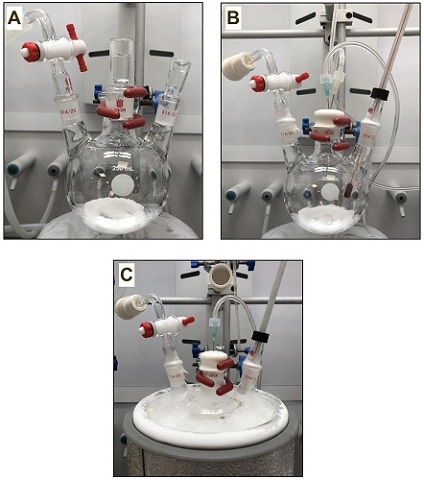
Figure 1. (A) Assembled reaction apparatus; (B) Reaction set-up with compound (1); (C) Reaction set-up prior to the addition of LiHMDS (Photos provided by checkers)
After stirring the pale-yellow solution for 30 min at -78±1 °C, allyl bromide (2.8 mL, 32 mmol, 3.0 equiv) (Note 7) is added dropwise via a plastic syringe fitted with a 21G needle over 12 min under an argon atmosphere. The internal temperature of the reaction mixture is maintained at -78±1 °C during the addition of allyl bromide. The pale-yellow reaction mixture is stirred at -78±1 °C for an additional 2.5 h. During this period, the internal temperature is maintained at -78±1 °C by periodic addition of dry ice to the bath. After 2.5 h at -78±1 °C, and completion of the reaction is confirmed by TLC analysis (Figure 2), the reaction mixture is quenched by the addition of saturated aqueous ammonium chloride solution (30 mL) (Note 8).
Figure 2. TLC of the crude reaction mixture, stained with iodine (A), KMnO4 (B), and ninhydrin (C). R: reaction mixture , C: co-spot, SM: starting material (1). Mobile phase: 25 % v/v ethyl acetate/hexanes (Photos provided by submitters and checkers)
The dry ice/acetone bath is removed, and the reaction mixture is allowed to warm to room temperature over the course of about 45 min (Figures 3A and 3B).
Figure 3. (A) Reaction mixture after removing the Dewar flask; (B) Reaction mixture at room temperature after 45 min (Photos provided by checkers)
The contents of the flask are then transferred to a 500-mL separatory funnel (Figure 4A). The phases are separated, and the aqueous phase is back-extracted with ethyl acetate (3 x 60 mL) The combined organic phases are finally washed with brine (20 mL) (Note 9)(Figure 4B).
Figure 4. (A) Biphasic mixture after transferring to separatory funnel without shaking; (B) Biphasic mixture after final washing with brine (Photos provided by checkers)
The crude mixture is then dried for 10 min over anhydrous Na2SO4 (10 g, Figure 5A) (Note 10), filtered through a fritted Büchner funnel (Note 11) under house vacuum (~75 mm Hg), and the filtrate is concentrated by rotary evaporation (36 °C, 30 mmHg), followed by high vacuum (20 °C, <1 mmHg) for 30 min to afford the crude mixture of (2) and (3) as a colorless oil (Figure 5B).
Figure 5. (A) Drying the organic phase over anhydrous Na2SO4; (B) Appearance of the crude product mixture after concentration (Photos provided by submitters)
A glass chromatography column (ID 5 cm × 30 cm ) is charged with silica gel (60 g) (Note 12) and is dry packed, eluting with 5% v/v ethyl acetate/ hexanes (Note 13) until the resulting silica gel column is homogeneous and cool to the touch. The crude oil is dissolved in CH2Cl2 (35 mL) (Note 14), then silica gel (5 g) is added (Note 12), and the solvent is evaporated by rotary evaporation (30 °C, 30 mmHg), followed by high vacuum (20 °C, <1 mmHg) for 30 min. The adsorbed silica gel is then loaded on the column, followed by a layer of sand (1 cm). The packed column is eluted with 300 mL of 5% v/v ethyl acetate/hexanes (Note 13), then 2000 mL of 10 % v/v ethyl acetate/ hexanes, followed by 800 mL of 20 % v/v ethyl acetate/hexanes. The eluates are collected in 25 x 180 mm test tubes totaling 47 fractions (Note 15). The fractions containing only compound (2) are combined and concentrated under reduced pressure on a rotary evaporator (36 °C, 30 mmHg), followed by high vacuum (20 °C, <1 mmHg, to afford compound (2) (Figure 6) as a colorless oil (2.86 g, 83% yield) (Notes 16, 17, 18, 19, and 20).

Figure 6. Purified compound (2) as a colorless oil. (Photo provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as in Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011); the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of the current procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
(S)-dimethyl 2-((tert-butoxycarbonyl)amino)pentanedioate,
tetrahydro-furan,
acetone, dry ice,
lithium bis(trimethylsilyl)amide,
allyl bromide,
ammonium chloride,
ethyl acetate,
hexanes,
dichloromethane,
sodium sulfate, silica gel and
deuterated chloroform.
2. The three-necked, round-bottomed flask containing a Teflon-coated egg-shaped magnetic stir bar were dried in an oven (105 ℃) for 16 h.
3.
(S)-Dimethyl 2-((tert-butoxycarbonyl)amino)pentanedioate (
1) was obtained from AK Scientific (Z4918-25g) as a white solid and used as received. The checkers purchased (
1) (> 98.0%) from TCI, which was used as received.
4. The checkers purchased HPLC grade
tetrahydrofuran from RCI Labscan Ltd., and passed it through a drying alumina column in a PureSolv MD 5 Solvent Purification System before use. The submitters used anhydrous
tetrahydrofuran obtained by distilling dry
tetrahydrofuran taken from MB-SPS (MBRAUN) over sodium in the presence of benzophenone and collecting it with a distillation trap (Figure 7).
Figure 7. Anhydrous tetrahydrofuran distillation set-up (Photo provided by submitters)
5. The Dewar flask was purchased from Pope Scientific Limited. The checkers purchased the Dewar flask from Chongqing Synthware Glass Inc.
6.
Lithium bis(trimethylsilyl)amide (1.0 M in
THF) in a Sure/Seal bottle was purchased from Sigma Aldrich (225770-100mL) as a light-yellow liquid and used as received. It was taken up from the Sure/Seal bottle via syringe under a positive pressure of argon. The checkers purchased
lithium bis(trimethylsilyl)amide (1.0 M in
THF) from Shanghai Macklin Biochemical Co., Ltd, which was used as received.
7.
Allyl bromide (ReagentPlus®, 99%, containing ≤1000 ppm propylene oxide as stabilizer) was purchased from Sigma Aldrich (A29585-100G) as a colorless liquid and used as received.
Allyl bromide was taken up from the amber-colored bottle via syringe under a flow of argon. The checkers purchased
allyl bromide (containing ≤1000 ppm propylene oxide as stabilizer, 98%) from Shanghai Aladdin Bio-Chem Technology Co., Ltd, which was used as received.
8. The checkers purchased
ammonium chloride (AR grade) from Dieckmann (Hong Kong) Chemical Industry Co. Ltd. which was used as received to prepare the saturated
ammonium chloride aqueous solution.
9. The checkers purchased
sodium chloride (AR grade) from Dieckmann (Hong Kong) Chemical Industry Co. Ltd. which was used as received.
10. The checkers purchased
sodium sulfate (anhydrous, AR grade) from Dieckmann (Hong Kong) Chemical Industry Co. Ltd. which was used as received.
11. The checkers used a medium porosity fritted Büchner funnel with a capacity of 150 mL.
12. The checkers purchased silica gel (0.040 - 0.063 mm) from Merck KGaA, which was used as received.
13. The checkers purchased
hexanes (GR grade) and
ethyl acetate (GR grade) from Duksan Pure Chemicals Ltd. which was used as received.
14. The checkers purchased GR grade
dichloromethane (99.8%) from RCI Labscan Ltd.
15. Thin layer chromatography (TLC) on silica gel 60 F254 (Aluminum TLC plate) eluting with 25 % v/v
ethyl acetate/
hexanes showing fractions 1-47 from column chromatography (Figure 8).
Figure 8. TLC fractions from column chromatography (Photo provided by checkers)
16. Analytical data for
dimethyl (2S,4S)-2-allyl-4-((tert-butoxycarbonyl)-amino)pentanedioate (
2) are as follows: IR (neat) 3367, 2979, 2955, 1713, 1642, 1506, 1367, 1159, 1049, 995, 779 cm
-1;
1H NMR (400 MHz, CDCl
3)
pdf δ: 5.70 (ddt,
J = 17.2, 10.2, 7.0 Hz, 1H), 5.12 - 5.00 (m, 2H), 4.95 (d,
J = 8.9 Hz, 1H), 4.35 (q,
J = 7.7 Hz, 1H), 3.72 (s, 3H), 3.66 (s, 3H), 2.57 (m,
J = 6.9 Hz, 1H), 2.41 - 2.24 (m, 2H), 2.00 (t,
J = 7.0 Hz, 2H), 1.43 (s, 9H);
13C NMR (150 MHz, CDCl
3)
pdf δ: 175.5, 172.8, 155.4, 134.3, 117.7, 80.1, 52.4, 52.1, 51.8, 41.8, 36.4, 33.7, 28.3; [α]
25D = + 22.8 (c 0.012, CHCl
3); HRMS (
m/z) calcd. for C
15H
25NO
6Na [M+Na]
+: 338.1580, found: 338.1577. Purity was assessed at 97.0% by qNMR
pdf using 1,3,5- trimethoxybenzene (Sigma-Aldrich, > 99.9%) as the internal standard.
17. A second reaction performed by the checkers on the same scale provided 3.03 g (88%) of compound (
2) at 97.3% purity, along with 0.179 g of a 55:45 mixture of (
3) and (
1).
18. The submitters purified the crude material by flash column chromatography, and isolated 2.92 g of pure (
2), 65 mg of a mixture of compound (
2) and side product (
3) and 203 mg of pure side product (
3) (Figure 9,
Note 19) as a pale, yellow oil.
Figure 9. Purified minor side product (3) as pale-yellow oil. (Photo provided by submitters)
19. Analytical data (provided by submitters) for
dimethyl (2S,4S)-2-allyl-4-((S)-N-(tert-butoxycarbonyl)-4-((tert-butoxycarbonyl)amino)-5-meth-oxy-5-oxopentanamido)pentanedioate (
3) are as follows: IR (neat) 2983, 2953, 1790, 1740, 1713, 1642, 1512, 1438, 1368, 1252, 1207, 1150, 1049, 1026, 917, 847, 754 cm
-1;
1H NMR
pdf (500 MHz, CDCl
3) δ: 5.72 (dddd,
J = 16.4, 10.4, 7.5, 6.1 Hz, 1H), 5.16 (d,
J = 8.6 Hz, 1H), 5.08 - 5.01 (m, 2H), 4.49 (dd,
J = 8.8, 6.9 Hz, 1H), 4.32 (t,
J = 7.3 Hz, 1H), 3.75 (s, 3H), 3.72 (s, 3H), 3.66 (s, 3H), 2.68 - 2.57 (m, 2H), 2.49 - 2.42 (m, 1H), 2.41 - 2.33 (m, 2H), 2.23 - 2.12 (m, 2H), 1.97 - 1.91 (m, 1H), 1.70 (ddd,
J = 13.3, 8.0, 6.9 Hz, 1H), 1.47 (s, 9H), 1.41 (s, 9H);
13C NMR
pdf (125 MHz, CDCl
3) δ 174.4, 173.2, 172.7, 171.9, 155.3, 149.3, 134.5, 117.6, 83.7, 79.9, 57.3, 52.8, 52.4, 52.4, 51.8, 42.1, 35.0, 30.0, 28.3, 27.8, 27.7, 26.8; [α]
25D = +14.4 (c 1.07, CHCl
3); HRMS (
m/z) calcd. for C
26H
42N
2O
11Na [M+Na]
+: 581.26863, found: 581.26853.
20. The submitters also separated by flash column chromatography 65 mg of the mixture of compound (
2) and side product (
3), obtained from the mixed fractions in the first chromatographic purification. Using a glass chromatography column with an inner diameter of 2 cm, and 5 g of silica gel, the column is eluted with 50 mL of 6% v/v
ethyl acetate/
n-hexane, 50 mL of 8% v/v
ethyl acetate/
n-hexane, then by 200 mL of 10 % v/v
ethyl acetate/
n-hexane. The eluate is collected in 13 x 100 mm test tubes (10 mL fractions) in 35 fractions (Figure 10). Fractions 11-18 contained pure (
2), which were concentrated by rotary evaporation (40 °C, 20 mmHg) and then under high vacuum (20 °C, 0.1 mmHg) for 30 min to obtain 20 mg pure (
2) for a total yield of 2.92 g + 0.02 g = 2.94 g (86%) of (
2) (Figure 6). Fractions 20-33 are concentrated by rotary evaporation (40 °C, 20 mmHg), then under high vacuum (20 °C, 0.1 mmHg) for 30 min to produce 37 mg of pure (
3) for a total yield of 203 mg + 37 mg = 240 mg (4%) of (
3) (Figure 9).
Figure 10. Thin layer chromatography (TLC) on silica gel 60 (SILICYCLE, glass backed TLC extra hard layer, 60Å) from the column chromatography of the mixture of product (2) and minor side product (3) (Photo provided by submitters)
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Most of the excitatory synapses of the mammalian CNS release glutamic acid as a chemical messenger.
2 Several CNS-related acute and chronic neurodegenerative disorders have been associated with excessive activation of glutamate receptors.
3 In order to delineate the roles of different glutamate receptors,
4 several conformationally constrained glutamate derivatives have been synthesized and tested for neurotropic activity. In this regard, enantiomerically pure 4- (or γ) substituted glutamates have garnered widespread attention.
It has been known for some time that the treatment of an
N-trityl L-glutamate dimethyl ester with a non-nucleophilic base such as LDA will generate the corresponding lithium enolate from the distal ester which can be reacted with benzyl chloroformate.
5 Extension of the reaction of
N-(9-phenylfluorenyl) L-glutamate diesters to include a variety of alkyl halides was reported by Rapoport and Koskinen.
6 They studied the effect of different non-nucleophilic bases on the diastereoselectivities of addition to give 4-substituted glutamates. North reported that the treatment of the lithium dianion of N-Cbz L-glutamate diesters with
allyl bromide or methyl iodide led to a 1:1 and 2:1 mixture respectively of 4-allyl and 4-methyl glutamates.
7 It was suggested that both faces of the dienolate were available to the electrophilic reagent. However, Gu reported that methylation of the dienolate of
N-
p-nitrobenzoyl dimethyl L-glutamate with methyl iodide afforded the 4-methylated glutamate with high
anti-selectivity.
8 The divergence in diastereoselectivities of additions in the reported cases may be attributed to subtle effects of the N-substituents and reaction conditions.
Following their studies on the
anti-selective γ-hydroxylation of the lithium dianion of dimethyl
N-Cbz L-glutamate with the Davis reagent (3-phenyl-
N-phenylsulfonyl oxaziridine),
9 Hanessian and Margarita reported the corresponding
anti-selective γ-allylation of dimethyl
N-Boc L-glutamate in absence of any additive.
10 The high
anti-selectivity was rationalized by a proposed transition state involving a lithium-coordinated 1,3-dianionic intermediate with a
Z(O)-type enolate geometry (Figure 11).
10,11Figure 11. Possible transition state models for high anti-selectivity
The Hanessian protocol starting with L-glutamic acid for the synthesis of
dimethyl 4(S)-allyl-N-Boc L-glutamate has been used in the context of the development of medicinally important compounds as well as the total synthesis of complex natural products.
12,13,14,15,16,17Herein, we report a revised procedure for the gram-scale synthesis of
dimethyl (2S,4S)-2-allyl-4-((tert-butoxycarbonyl)amino)pentanedioate (
2) from
dimethyl N-Boc L-glutamate (
1) using a highly
anti-selective allylation of the lithium dienolate. In the present version, we also isolated the self-condensed product (
3) (4% yield), which we had not reported in our original procedure.
10 The formation of the by-product (
3) varies with the reaction conditions, and is increased when using (a) 2.2 equiv of
LiHMDS, 3.0 equiv of
allyl bromide and stirring at -78 °C for 4.5 h, then NH
4Cl (8.1% of 3 obtained), (b) 2.3 equiv of
LiHMDS, 3.0 equiv of
allyl bromide and stirring at -78 °C for 3 h, then NH
4Cl (8.3% of 3 obtained). The experimental protocol is also applicable to the synthesis of the
N-Cbz analog, as well as other 4-substituted derivatives of L-glutamic acid using methallyl bromide and cinnamyl bromide as electrophiles (Table 1).
Table 1. Substrate scope in the synthesis of dimethyl 4(S)-substituted N-Boc L-glutamates
Appendix
Chemical Abstracts Nomenclature (Registry Number)
(S)-Dimethyl 2-((tert-butoxycarbonyl)amino)pentanedioate; (1) (59279-60-6)
Lithium bis(trimethylsilyl)amide (1M in THF); (4039-32-1)
Allyl bromide; (106-95-6)

|
Akash Mishra obtained his Ph.D. in 2018 at Banaras Hindu University, under the supervision of Prof. Ganesh Pandey. He moved to Ulsan National Institute of Science and Technology (UNIST), for post-doctoral research with Prof. Cheol-Min Park. He is currently working as a post-doctoral fellow in the group of Prof. Stephen Hanessian at Université de Montréal. His research interests involve asymmetric total synthesis of natural products, visible light photoredox catalysis, and synthesis of biologically active compounds having medicinal importance. |

|
Stephen Hanessian holds the Ionis Pharmaceuticals and Servier/NSERC Senior Industrial Research Chair at Université de Montréal. He is also Distinguished Professor in the Department of Pharmaceutical Sciences at the University of California, Irvine USA. His research interests cover a wide cross section of areas covering organic, bioorganic, and medicinal chemistry. |

|
Qin Han Teo was born in Selangor, Malaysia. She received her BSc. degree in Chemical Technology from The Hong Kong Polytechnic University. As an undergraduate, she also worked with Prof. Ian Baxendale in a research exchange at Durham University, UK. She continued her studies at the University of Hong Kong, where she is currently a third-year graduate student studying (4+3) cycloadditions in Prof. Pauline Chiu's laboratory. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved