Org. Synth. 1993, 71, 200
DOI: 10.15227/orgsyn.071.0200
TITANIUM-MEDIATED ADDITION OF SILYL DIENOL ETHERS TO ELECTROPHILIC GLYCINE: 4-KETOPIPECOLIC ACID HYDROCHLORIDE
[Pipecolic acid, 4-oxo-, hydrochloride]
Submitted by Clarisse Mühlemann, Peter Hartmann, and Jean-Pierre Obrecht
1.
Checked by Eugene Ho and David L. Coffen.
1. Procedure
A. 2-Bromo-N-Boc-glycine tert-butyl ester. In a 1-L, round-bottomed flask are placed 20.0 g (0.0865 mol) of N-Boc-glycine tert-butyl ester (Note 1) and 16.2 g (0.0912 mol) of N-bromosuccinimide (Note 2). Carbon tetrachloride (350 mL, (Note 3)) is added, the flask is connected to a clean rotatory evaporator (Note 4) and the apparatus is flushed with argon. The flask is cooled, while being rotated, by means of a water bath and is irradiated with two 150-W tungsten lamps (Note 5) for 1 hr. The colorless solution becomes dark red and a precipitate forms. The suspension is filtered through a Schlenk tube and the carbon tetrachloride (CCl4) is evaporated under reduced pressure. The remaining yellowish oil is employed in the next step without purification (Note 6).
B. tert-Butyl [1-(tert-butoxycarbonyl)-3-oxo-4-pentenyl]carbamate. The crude bromination product from the previous step is taken up in 240 mL of dry tetrahydrofuran (THF) (Note 7) and transferred to a 1000-mL flask equipped with a stirrer, thermometer, dropping funnel, and argon inlet. The solution is cooled to −78°C and a solution of 42 g (0.20 mol) of dichlorodiethoxytitanium [TiCl2 (OEt)2] in 80 mL of dry THF (Note 8) is added at such a rate that the internal temperature does not exceed −72°C. When the addition is complete, the reaction mixture is stirred at −78°C for 10 min and then 24 g (0.170 mol) of 2-trimethylsiloxybutadiene (Note 9) in 100 mL of THF is added dropwise, causing only a slight increase in temperature (−72°C). The reaction mixture is allowed to warm to room temperature overnight and poured into 700 mL of ice-cooled, saturated sodium bicarbonate solution. After filtration through Celite, the aqueous phase is extracted with three 200-mL portions of ether. The combined organic layers are washed twice with water, dried over magnesium sulfate (MgSO4), filtered, and concentrated. The remaining dark oil (29 g) is subjected to flash chromatography (20-cm column diameter, ether/hexane 1:3); 8.44–8.73 g (33–36%) (Note 10) of the product is obtained as a slightly yellowish oil (Note 11).
C. 4-Ketopipecolic acid hydrochloride. tert-Butyl [1-(tert-butoxycarbonyl)-3-oxo-4-pentenyl]carbamate, 8.73 g (0.0308 mol), is dissolved in 280 mL of an ice-cooled, saturated solution of hydrogen chloride in ether. The solution is kept without stirring at room temperature overnight. The resulting suspension is filtered and the filter cake is immediately washed with dry ether (Note 12). The washing with ether is repeated four times and, after drying under reduced pressure, 5.48 g (99%) of 4-ketopipecolic acid hydrochloride is obtained as a colorless powder, mp 139–142°C dec (Note 13).
2. Notes
1.
Boc-Glycine tert-butyl ester can be prepared by treatment of
glycine tert-butyl ester hydrochloride (Aldrich Chemical Company, Inc.) with
di-tert-butyl dicarbonate (Fluka Chemical Corporation) and
triethylamine in THF.
22.
N-Bromosuccinimide was purchased from Fluka Chemical Corporation, recrystallized from water, and dried well in a
vacuum desiccator.
3.
Carbon tetrachloride is toxic and should only be handled in a
well-ventilated hood.
4.
The rotatory evaporator was washed with
ethanol and ether. Bromination on a smaller scale can be carried out in a
three-necked, round-bottomed flask with a thermometer, argon inlet, and stirring bar. External cooling with a water bath to keep the internal temperature between 15° and 20°C is important. The use of more concentrated solutions should be avoided, since dimerization instead of bromination becomes the dominant reaction.
5.
The water bath was coated with
aluminum foil in order to increase the efficiency of the irradiation.
6.
TLC (
ethyl acetate/
hexane 1:3;
vanillin/concd. H
2SO
4/heat) reveals complete consumption of the starting material and only small amounts of impurities. The crude product is stable for several weeks at −20°C under
argon.
27.
THF was distilled from
potassium and benzophenone.
8.
Tetraethyl orthotitanate, 22.9 g (0.100 mol), (Fluka Chemical Corporation, bulb-to-bulb-distilled at
110–115°C/0.1 mm) is dissolved in
80 mL of dry THF.
Titanium chloride (TiCl4) (Fluka Chemical Corporation), 19.0 g (0.100 mol, distilled at
136°C/atmospheric pressure) was added dropwise while cooling with an
acetone/dry ice bath to keep the temperature below 0°C. Alternatively, TiCl
4 may be added to a solution of Ti(OEt)
4 (obtained from Aldrich Chemical Company, Inc.) in
hexane at 0°C; the solvent is evaporated and replaced by THF.
39.
Trimethylsiloxybutadiene was purchased from Petrarch Systems, Inc., and employed without further purification. The checkers attempted to use the more stable
triethylsiloxybutadiene without success.
10.
In an experiment on one tenth the scale, the yield was
57%.
411.
The physical properties are as follows:
1H-NMR (CDCl
3) δ: 1.44 (s, 18 H), 3.08 (dd, 1 H, J = 4 and 18), 3.28 (dd, 1 H, J = 4 and 18), 4.45 (m, 1 H), 5.48 (d, 1 H, J = 8, N-H), 5.91 (dd, 1 H, J = 2 and 10), 6.25 (dd, 1 H, J = 2 and 18), 6.34 (dd, 1 H, J = 10 and 18); IR (CHCl
3) cm
−1: 3430, 3000, 2980, 2930, 1740, 1720, 1710, 1630, 1500.
12.
When the filter cake contains
hydrogen chloride, it is very hygroscopic. It should therefore be covered immediately with dry
ether after the ethereal
hydrogen chloride solution has been aspirated.
13.
The physical properties are as follows:
1H NMR (MeOD) δ: 3.05 (dt, 2 H, J = 2 and 6), 3.20 (dd, 1 H, J = 7 and 20), 3.29 (dd, 1 H, J = 4 and 20), 3.79 (t, 2 H, J = 6), 4.30 (dd, 1 H, J = 4 and 7); IR (nujol) cm
−1: 3300–2300 broad, 3060, 2960, 2920, 2860, 1740, 1725, 1600, 1570.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
N-Boc-2-Bromoglycine tert-butyl ester (
1), introduced by Steglich and co-workers, is a versatile synthon for electrophilic
glycine,
5 an important tool in the synthesis of non-proteinogenic amino acids.
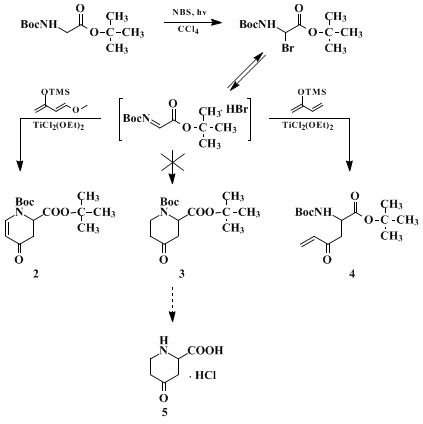
Elimination of HBr leads to an acylimino acetate that should be able to undergo an aza-Diels–Alder reaction with dienes to give pipecolic acid derivatives not readily accessible by other methods. Indeed,
1, in the presence of TiCl
2(OEt)
2, reacts with Danishefsky's diene between −78°C and room temperature to give the cyclic compound
2 in 72% yield. In a thermal reaction of
2-trimethylsiloxybutadiene with another electrophilic
glycine equivalent, Jung and co-workers
6 isolated a cyclic product of type
3. Under the reaction conditions described here, the reaction product is not
3 but the enone
4, which by itself is an interesting bifunctional intermediate. However, upon deprotection the anticipated ring closure takes place in a very clean fashion. Pure
4-ketopipecolic acid hydrochloride crystallizes out of the ethereal
hydrogen chloride solution in quantitative yield, which illustrates the advantage of the use of
1 in amino acid synthesis, i.e., the ease of deprotection, often a critical step.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
ethanol (64-17-5)
hydrogen chloride (7647-01-0)
ethyl acetate (141-78-6)
ether (60-29-7)
sodium bicarbonate (144-55-8)
carbon tetrachloride (56-23-5)
aluminum (7429-90-5)
Benzophenone (119-61-9)
potassium (7440-09-7)
Glycine (513-29-1)
magnesium sulfate (7487-88-9)
vanillin (121-33-5)
Tetrahydrofuran (109-99-9)
N-bromosuccinimide (128-08-5)
hexane (110-54-3)
titanium chloride (7550-45-0)
triethylamine (121-44-8)
argon (7440-37-1)
2-trimethylsiloxybutadiene (38053-91-7)
Di-tert-butyl dicarbonate (24424-99-5)
4-Ketopipecolic acid hydrochloride,
Pipecolic acid, 4-oxo-, hydrochloride (99979-55-2)
2-Bromo-N-Boc-glycine tert-butyl ester,
N-Boc-2-Bromoglycine tert-butyl ester
N-Boc-glycine tert-butyl ester,
Boc-Glycine tert-butyl ester (111652-20-1)
tert-Butyl [1-(tert-butoxycarbonyl)-3-oxo-4-pentenyl]carbamate (117833-62-2)
dichlorodiethoxytitanium (3582-00-1)
glycine tert-butyl ester hydrochloride (27532-96-3)
Tetraethyl orthotitanate (3087-36-3)
Trimethylsiloxybutadiene
triethylsiloxybutadiene
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved