Checked by Eugene Ho and David L. Coffen
Rechecked by Yasuhiro Shimamoto, Heemal Dhanjee and John L. Wood
1. Procedure
B. tert-Butyl [1-(tert-butoxycarbonyl)-3-oxo-4-pentenyl]carbamate . The crude bromination product from the previous step is taken up in dry
tetrahydrofuran (120 mL) (
Note 7) and transferred to a 500 mL 3-necked round-bottomed flask equipped with an egg-shaped, teflon-coated magnetic stir bar (3.0 cm x 1.5 cm), a rubber septum with thermocouple temperature probe (Neck 1), a 100-mL dropping funnel capped with a rubber septum (Neck 2), and a nitrogen inlet (Neck 3) (Figure 2). The solution is cooled to -78 °C in a dry ice-acetone bath and a solution of
dichlorodiethoxytitanium [
TiCl2(OEt)2] (21.0 g, 100 mmol, 2.3 equiv) in
THF (40 mL, 2.5 M) (
Note 8) is added slowly to ensure the internal temperature does not exceed -72 °C. When the addition is complete, the reaction mixture is stirred for 10 min at which point a solution of
2-trimethylsiloxybutadiene (12.0 g, 85 mmol, 2.0 equiv) (
Note 9) in
THF (50 mL, 1.7 M) is added dropwise, resulting in a slight increase in temperature (to -72 °C). The reaction mixture is allowed to warm to room temperature. After 12 h, the mixture is poured into 350 mL of ice-cooled, saturated
sodium bicarbonate solution and is filtered through a pad of Celite (60 g) using a fritted filter funnel (10 cm diameter, Fine). The celite is washed with
diethyl ether (2 x 100 mL). The filtrate is transferred to a 1 L separatory funnel. The layers are separated, and the aqueous phase is extracted with
diethyl ether (2 x 100 mL). The combined organic layers are washed with brine (1 x 100 mL), dried over anhydrous
sodium sulfate (
Na2SO4), filtered through a cotton plug and collected in a 1-L round-bottomed flask. The Na
2SO
4 is rinsed with
diethyl ether (2 x 20 mL). The solvent is removed under reduced pressure by rotary evaporation (50 mmHg, 30 °C). The dark crude oil is purified by column chromatography. A fritted chromatography column (3.0 cm diameter) is dry-packed with silica gel (20 cm height, 50 g) (
Note 10) and then wetted with hexanes under air pressure. The crude oil is loaded directly onto the column and eluted with 1 L of 10:1 hexane/ethyl acetate. The eluent is collected in 25 mL fractions and monitored by TLC (Product R
f = 0.11, hexane/ethyl acetate: 10/1) (Notes
6 and
11). The fractions are combined in a 1-L round-bottomed flask and concentrated under reduced pressure by rotary evaporation (50 mmHg, 30 °C) to give
tert-butyl [1-(tert-butoxycarbonyl)-3-oxo-4-pentenyl] carbamate as a yellowish oil (2.82 g, 22%) (
Note 12).
Figure 2. Reaction Set-up for Step B
2. Notes
1. Unless otherwise noted, all glassware and stir bars were flame dried under vacuum, cooled to room temperature under vacuum, and placed under nitrogen atmosphere.
2.
N-Boc-glycine tert-butyl ester was prepared as follows: A 1 L one-necked round-bottomed flask was equipped with an egg shaped stir bar (3.0 cm x 1.5 cm) and capped with a rubber septum.
Boc-Gly-OH (99%, purchased from Oakwood Chemical and used as received) (30.2 g, 171 mmol, 1.0 equiv) and
DCM (600 mL, 0.29 M) were added. The solution was cooled in ice-water bath, and then
tBuOH (15.3 g, 202 mmol, 1.2 equiv),
DMAP (1.5 g 12 mmol, 0.07 equiv) and
DCC (41.3 g, 200 mmol, 1.2 equiv) were added. The reaction mixture was stirred for 12 h, and the precipitate was filtered using a fritted filter funnel (4.5 cm in diameter, Fine). The filtrate was concentrated under reduced pressure by rotary evaporation (50 mmHg, 30 °C water bath), and the residue was purified by flash column chromatography on silica gel (8.0 cm diameter, 20 cm height of silica gel (400 g)) (
Note 10). Hexanes/ethyl acetate: 10/1 (100 mL) and hexanes/ethyl acetate: 5/1 (2000 mL) were used as eluents, which were collected in 50 mL fractions (
Note 11). The desired product (R
f = 0.40, hexane/ethyl acetate: 5/1) containing minor impurities was obtained (33 g). The material was recrystallized twice from hexanes. Hexanes (100 mL) was added and heated to 60 °C so as to dissolve all the solids. The solution was left to cool slowly to room temperature and subsequently placed in a -23 °C freezer for approximately 12 h. The crystals were then collected on a Kiriyama filter. The recrystallization was repeated a second time to provide
N-Boc-glycine tert-butyl ester (16.6 g, 71.8 mmol, 41.9%yield)
3.
N-Bromosuccinimide was purchased from Sigma Aldrich, recrystallized from water, and dried in a vacuum desiccator (1.0 mmHg, 25 °C).
4.
Chlorobenzene was purchased from Acros Organics and used without further purification. The submitters and previous checkers used CCl
4. Difficulties in obtaining CCl
4 led the current checkers to make this solvent change.
5. The water bath was coated with aluminum foil.. Foil was wrapped around the entire reaction assembly in order to increase the efficiency of the irradiation. The checkers used a 300W tungsten lamp while the submitters used a 150W tungsten lamp.
6. TLC (ethyl acetate/hexane 1:3; visualized with vanillin/concd. H
2SO
4/heat) (
Note 10) reveals complete consumption of the starting material (starting material R
f = 0.47) and only small amounts of impurities are observed by
1H NMR
pdf analysis. The crude product is stable for several weeks at −20 °C under argon.
7. Non-stabilized
THF was purchased from Fisher Scientific and passed through two packed columns of neutral alumina in a solvent purification system manufactured by SG Water U.S.A., LLC.
8. The equivalents of reagents used in this reaction are based on the amount of starting material used in step A of the reaction sequence.
Tetraethyl orthotitanate, (11.5 g, 50 mmol) was purchased from Sigma-Aldrich and distilled at 110-115 °C at 0.1 mmHg. The distilled material is dissolved in dry
THF (40 mL) (
Note 7).
Titanium chloride (
TiCl4) (9.5 g, 50 mmol) was purchased from Sigma-Aldrich and distilled at 136 °C under atmospheric pressure. The titanium tetrachloride was added dropwise to the solution of
tetraethyl orthotitanate while cooling in an acetone/dry ice bath.
9.
Trimethylsiloxybutadiene was purchased from Oakwood Chemical, and employed without further purification.
10. Silica gel SilicaFlash
® F60 (40-63 µ / 230-500 mesh) was purchased from Silicycle. Glass-backed extra hard layer TLC plates, 60 Å (250 µm thickness) were also purchased from Silicycle containing F-254 indicator.
11.
Ethyl acetate and hexanes (all ACS Grade) used in chromatography were purchased from Fisher Scientific and used as received.
12. The submitters obtained a 33-36% yield over this two-step sequence, and the checkers note that the change in solvent from CCl
4 to
chlorobenzene and a change in the lamp from 150 W to 300 W could account for this difference in yield. The physical properties are as follows:
1H NMR
pdf(CDCl
3) δ: 1.42 (s, 18 H), 3.07 (dd, 1 H,
J = 4.8, 17.6 Hz), 3.28 (dd, 1 H,
J = 4.8, 17.6 Hz), 4.44 (m, 1 H), 5.47 (d, 1 H,
J = 8.0 Hz, N-H), 5.90 (dd, 1 H,
J = 1.2, 10.4 Hz), 6.24 (dd, 1 H,
J = 1.2, 17.6 Hz), 6.33 (dd, 1 H,
J = 10.4, 18.0 Hz);
13C NMR
pdf(CDCl
3) δ: 27.8, 28.3, 41.5, 50.1, 82.1, 129.3, 136.2, 155.6, 170.2, 198.3; IR (ATR) cm
−1: 3370, 2980, 2930, 1710, 1620, 1490. HRMS (+ESI) calcd for C
15H
25NO
5 (M+Na) 322.1630. Found 322.1631.
13. This compound is very hygroscopic.
14.
Hydrogen chloride in
diethyl ether was prepared by bubbling
hydrogen chloride into
diethyl ether for 2 h.
Hydrogen chloride was generated from treatment of
NaCl with
H2SO4.
15. The physical properties are as follows:
1H NMR
pdf(CD
3OD) δ: 3.05 (dt, 2 H,
J = 1.2, 6.4 Hz), 3.20 (dd, 1 H
, J = 6.8, 18.8 Hz), 3.29 (dd, 1 H,
J = 4.0, 19.6 Hz), 3.79 (t, 2 H,
J = 6.4 Hz), 4.30 (dd, 1 H,
J = 4.0, 6.8 Hz);
1H NMR
pdf(400 MHz, D
2O) δ: 3.11 (t, 2H,
J = 6.0 Hz), 3.32-3.34 (m, 2H), 3.81 (t, 2H,
J = 6.0 Hz), 4.27 (dd, 1H,
J = 4.4, 6.0 Hz);
13C NMR
pdf(100 MHz, CD
3OD) δ: 28.0, 38.7, 42.9, 45.7, 48.4, 170.9, 205.6;
13C NMR
pdf(100 MHz, D
2O) δ: 37.9, 41.7, 44.1, 48.5, 171.4, 207.8; IR (ATR) cm
−1: 3300-2300 broad, 1720, 1580, 1400. HRMS (+ESI) calcd for C
6H
11Cl
2NO
3 (M-Cl) 180.0422. Found 180.0424. mp = 116 - 118 °C (decomp).
16. In an effort to confirm the structure, the checkers employed the illustrated procedure to convert
1-carboxy-5-chloro-3-oxopentan-1-aminium chloride to
2-tert-butyloxycarbonylamino-6-chloro-4-hexanolide, a compound which had been stereoselectively prepared previously by Hesse.
2
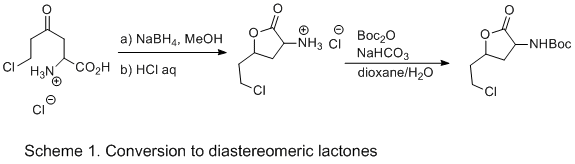
In a 100 mL one-necked round-bottomed flask equipped with a 2.0 cm x 0.5 cm rod shaped stir bar, 1-carboxy-5-chloro-3-oxopentan-1-aminium chloride (500 mg, 2.3 mmol, 1.0 equiv) is dissolved in MeOH (10 mL, 0.23 M) and cooled in an ice-water bath. NaBH4 (130 mg, 3.5 mmol, 1.5 equiv) is added and the reaction stirred for 2 h. The solvent is removed under reduced pressure by rotary evaporation (50 mmHg, 30 °C water bath). The crude alcohol is then dissolved in 1 M aq. HCl (20 mL) and stirred for 2 h after which the solvent is removed under reduced pressure (1.0 mmHg, 25 °C water bath). The lactonized ammonium salt is subsequently dissolved in dioxane/H2O (1/1) (50 mL), and treated with NaHCO3 (1.9 g, 23 mmol, 10 equiv) followed by Boc2O (610 mg, 2.8 mmol, 1.2 equiv). The reaction mixture is stirred for 12 h, after which time the reaction mixture is extracted with ethyl acetate (3 x 30 mL). The combined organic layers are washed with brine (1 x 30 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure by rotary evaporation (50 mmHg, 30 °C water bath). The residue is purified by flash column chromatography on silica gel. Hexanes/ethyl acetate:3/1 (500 mL) is used as the eluent and collected in 10 mL fractions. The fractions were monitored by TLC (Rf = 0.18, hexane/ethyl acetate: 3/1). The first fractions were concentrated to give 12.3 mg of the cis-isomer (2.0% over 3 steps). The last fractions were concentrated under reduced pressure to give 10.3 mg of the trans-isomer (1.7% over 3 steps), and the middle fractions were concentrated under reduced pressure to provide 182 mg of a 47:53 cis:trans (30% over 3 steps). In all cases was the 2-tert-butyloxycarbonylamino-6-chloro-4-hexanolide obtained as a white solid. cis: 1H NMR pdf(CDCl3) δ: 1.45 (s, 9 H), 1.88 (q, 1 H, J = 12.0 Hz), 2.05-2.14 (m, 1 H), 2.17-2.26 (m, 1 H), 2.84-2.93 (m, 1 H), 3.66-3.72 (m, 2 H), 4.35-4.47 (m, 1 H), 4.62-4.69 (m, 1 H), 5.09 (bs, 1 H); 13C NMR pdf(100 MHz, CDCl3) δ: 28.2, 36.4, 38.0, 40.3, 51.4, 74.6, 80.7, 155.3, 174.3; IR (ATR) cm−1: 3420, 2980, 2930, 1770, 1700, 1510. trans: 1H NMR pdf(CDCl3) δ: 1.45 (s, 9 H), 1.97-2.07 (m, 1 H), 2.12-2.20 (m, 1 H), 2.34-2.53 (m, 2 H), 3.66 (dd, 2H, J = 5.2, 7.6 Hz), 4.32-4.45 (m, 1 H), 4.84-4.93 (m, 1 H), 5.09 (bs, 1 H); 13C NMR (100 MHz, CDCl3) δ: 28.2, 34.3, 38.1, 40.3, 49.2, 74.3, 80.9, 155.4, 174.7; IR (ATR) cm−1: 3380, 2980, 2930, 1780, 1670, 1520, HRMS (+ESI) calcd for C11H18ClNO4 (M+Na) 286.0822. Found 286.0819. Melting Point: 121.4-123.1 °C.
To unambiguously confirm the identity of the lactone product, the checkers prepared single crystals suitable for X-Ray crystallographic analysis using the trans-isomer (Figure 3). Single crystals are grown from a DCM solution of the trans-isomer by vapor diffusion into hexanes. Accordingly, 10 mg of trans-2-tert-butyloxycarbonylamino-6-chloro-4-hexanolide are placed in a 1.5 dram vial and dissolved in a minimal amount of DCM (ca. 0.5 mL). This vial is placed into a larger 50 mL jar containing a volume of hexanes such that the meniscus is slightly above that of the DCM in the inner vial. The larger vial is sealed and let stand at room temperature to produce colorless cube crystals (ca. 6 h). Single crystal X-ray diffraction data were collected on a Bruker Apex II-CCD detector using Mo-Kα radiation (λ = 0.71073 Å).
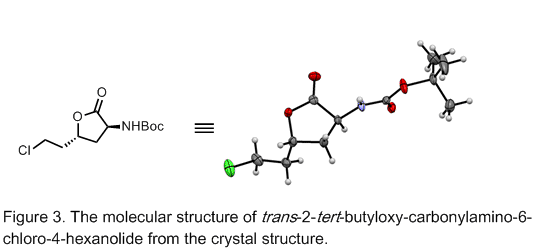
Crystals are removed from the mother liquor and coated in an inert perfluoro-polyether oil, mounted on micromounts then placed in a cold stream of N2. Structures were solved and refined using SHELXTL (Table 1). These data have been deposited with the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication no. CCDC 1435009 and copies can be obtained in the supporting information or free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
3. Discussion
Although it is noteworthy that spectral data obtained for the product derived from
1 differs from that reported for
2 in papers published by Burger in 1995 and 1996,
4 positive proof for the structural reassignment was sought via elemental composition data. Mass spectrometry was indicative of structure
3 but efforts to obtain an acceptable elemental analysis furnished results outside the acceptable ± 0.4% window, although the data obtained from multiple EA attempts were all more consistent with structure
3 than for
2.
5 Given the inability to obtain satisfactory EA data the checkers turned to chemical correlation as a means of providing compelling evidence for structure
3 and thus attempted to convert
3 to lactones (±)-
6 and (±)-
7, the former of which had been previously prepared in enantioenriched form via an unrelated route by Hesse (Scheme 2).
2
Although, spectral data obtained by the checkers for (±)-
6 was similar to that reported for (+)-
6 by Hesse, there were clear discrepancies in both the
1H and
13C NMR data. Thus, the checkers turned to X-ray crystallography in order to confirm the structure. To this end
trans-lactone (±)-
7 was found to furnish crystals suitable for X-ray analysis and the derived structure provided convincing proof that the compound
3 is precursor to
7 and thus does indeed arise upon treatment of
1 with HCl in Et
2O.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Glycine (513-29-1)
N-Bromosuccinimide (128-08-5)
Titanium chloride (7550-45-0)
Triethylamine (121-44-8)
2-Trimethylsiloxybutadiene (38053-91-7)
Di-tert-butyl dicarbonate (24424-99-5)
4-Ketopipecolic acid hydrochloride; Pipecolic acid, 4-oxo-, hydrochloride (99979-55-2)
2-Bromo-N-Boc-glycine tert-butyl ester, N-Boc-2-Bromoglycine tert-butyl ester
N-Boc-glycine tert-butyl ester, Boc-Glycine tert-butyl ester (111652-20-1)
tert-Butyl [1-(tert-butoxycarbonyl)-3-oxo-4-pentenyl]carbamate (117833-62-2)
Dichlorodiethoxytitanium (3582-00-1)
Glycine tert-butyl ester hydrochloride (27532-96-3)
Tetraethyl orthotitanate (3087-36-3)
Trimethylsilyloxybutadiene (38053-91-7)
N-Boc-glycine (4530-20-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved