Org. Synth. 2000, 77, 162
DOI: 10.15227/orgsyn.077.0162
SYNTHESIS OF 2'-DEOXYRIBONUCLEOSIDES: β-3',5'-DI-O-BENZOYLTHYMIDINE
[
Thymidine, 3',5'-dibenzoate
]
Submitted by Daniel R. Prudhomme, Minnie Park, Zhiwei Wang, Jason R. Buck, and Carmelo J. Rizzo
1
.
Checked by Gilles Chambournier, Jane Djung, and David J. Hart.
1. Procedure
A.
1,3,5-O-Tribenzoyl-2-O-[(3-trifluoromethyl)benzoyl]-α-D-ribofuranose
. In an
oven-dried, 500-mL, two-necked, round-bottomed flask equipped with a
magnetic stir bar and a
rubber septum is placed
1,3,5-tri-O-benzoyl-α-D-ribofuranose (5.0 g, 10.8 mmol)
(Note 1) and
2,6-lutidine (1.4 g, 13.0 mmol)
(Note 2) in
200 mL of dry dichloromethane
(Note 3) under an
argon atmosphere. The reaction mixture is cooled to 0°C and
3-(trifluoromethyl)benzoyl chloride (3.3 g, 16.2 mmol)
(Note 4) is added dropwise to the stirred solution over 30 min. After the addition, the reaction mixture is warmed to room temperature and stirred overnight. The reaction is quenched with
80 mL of aqueous saturated sodium bicarbonate
, the phases are separated and the aqueous phase is extracted with
dichloromethane (2 × 200 mL). The combined organic extracts are washed with
brine (2 × 100 mL) and dried over anhydrous
magnesium sulfate
. After filtration, the solvent is removed under reduced pressure to give a yellow oil. Purification by flash column chromatography (
140 g of silica gel)
(Note 5) and eluting with
25%
ethyl acetate
in hexanes gives a white solid that can be recrystallized from
ethyl acetate/
hexanes to yield
1,3,5-O-tribenzoyl-2-O-[(3-trifluoromethyl)benzoyl]-α-D-ribofuranose
(
5.62 g,
82% yield) as white crystals, mp
109-111°C (lit.
2 mp
102°C)
(Note 6).
B.
2,4-Bis(trimethylsilyloxy)-5-methylpyrimidine
. In an
oven-dried, 1-L, round-bottomed flask is placed
thymine (12.6 g, 0.1 mol)
(Note 1) and
chlorotrimethylsilane
(Note 7) in
300 mL of benzene
(Note 8) under an
argon atmosphere. To the stirred suspension is added
triethylamine (20.2 g, 0.2 mol)
(Note 9) dropwise via a
dropping funnel over 1 hr. After the addition is complete, the reaction is stirred overnight. The precipitated
triethylammonium chloride
and unreacted
thymine are removed by suction filtration
(Note 10) and the solid is washed with dry
benzene (2 × 30 mL). The solvent is then removed with a
rotary evaporator. The resulting viscous oil is distilled under reduced pressure (bp
94-97°C at 9 mm, lit.
3 bp
124°C at 14 mm) to give
2,4-bis(trimethylsilyloxy)-5-methylpyrimidine
(
24.0 g,
89% yield). The product solidifies upon standing overnight, mp
73-75°C (lit.
3 mp
63-65°C)
(Note 11).
C.
3',5'-Di-O-benzoyl-2'-O-[(3-trifluoromethyl)benzoyl]-5-methyluridine
. In an oven-dried, 250-mL, round-bottomed flask equipped with a magnetic stir bar and a rubber septum is placed
1,3,5-tri-O-benzoyl-2-O-[(3-trifluoromethyl)benzoyl]-α-D-ribofuranose (5.6 g, 8.8 mmol) and
2,4-bis(trimethylsilyloxy)-5-methylpyrimidine (2.16 g, 7.1 mmol) in
100 mL of acetonitrile
(Note 12). At this stage, the reaction may appear as a white suspension (Note 13). The stirred reaction mixture is cooled to 0°C and
tin(IV) chloride (13.75 g, 52.8 mmol)
(Note 14) is added dropwise over 15 min with vigorous stirring. Upon addition of tin(IV) chloride, the reaction becomes homogeneous. The reaction mixture is warmed to room temperature and stirred for 6 hr. The reaction is then quenched by pouring the mixture into a 1-L separatory funnel containing
250 mL of aqueous saturated sodium bicarbonate
and 200 g of ice. Upon quenching the reaction, voluminous white precipitates appear.
Ethyl acetate (200 mL) is added, the separatory funnel is cautiously swirled, and the layers are allowed to separate. The aqueous layer, which now appears milky white, is further extracted with
ethyl acetate (2 × 100 mL). The combined extracts are washed with
brine (2 × 200 mL) and dried over anhydrous
sodium sulfate
. After filtration, the solvent is removed under reduced pressure to give a yellow oil. Purification by flash column chromatography (
100 g of silica gel) (Note 5) eluting with
25%
ethyl acetate
in hexanes gives
3',5'-di-O-benzoyl-2'-O-[(3-trifluoromethyl)benzoyl]-5-methyluridine
as a white solid (3.52 g, 62% yield), mp 92-95°C
(Note 15), (Note 16).
D.
3',5'-Di-O-benzoylthymidine
. In a
500-mL photochemical reaction vessel,
(Note 17) equipped with a magnetic stir bar, is placed
3',5'-di-O-benzoyl-2'-O-[(3-trifluoromethyl)benzoyl]-5-methyluridine (3.82 g, 6.0 mmol),
magnesium perchlorate hexahydrate (0.46 g, 1.4 mmol) (Note 1)
and
9-ethyl-3,6-dimethylcarbazole (200 mg, 0.9 mmol
; for preparation see: Buck, J. P.; Park, M.; Wang, Z.; Prudhomme, D. R.; Rizzo, C. J.
Org. Synth.
1999, 77, 153) in
500 mL of 9:1 2-propanol/water
(Note 18). The solution is degassed by bubbling
argon through the solution for 1 hr using a syringe needle. The photochemical immersion well, equipped with a
Pyrex filter is fitted into the reaction vessel and made air tight by lightly greasing the ground glass joints; an
argon atmosphere is maintained. The reaction is cooled with an
ice bath
(Note 19) and photolyzed with a
Hanovia 450W, medium pressure mercury lamp through a Pyrex filter for 1 hr
(Note 20). The reaction mixture is transferred to a
1-L flask and the solvent is removed with a rotary evaporator. The residue is dissolved in
ethyl acetate (150 mL), washed with aqueous saturated
sodium bicarbonate (2 × 30 mL), then
brine (30 mL), and the organic phase is dried over
magnesium sulfate. After filtration, the solvent is removed under reduced pressure to give a yellow oil. Purification by flash column chromatography (
150 g of silica gel)
(Note 5) eluting with
50%
ethyl acetate
in hexanes gives
3',5'-di-O-benzoylthymidine
(
1.43 g,
53% yield), mp
195-196°C (lit.
4 mp
194-195°C)
(Note 21), and
5'-O-benzoyl-3'-deoxythymidine
(
0.32 g,
16% yield), mp
71-74°C (lit.
5 mp
57-59°C)
(Note 22), and recovered starting material (
0.41 g,
11% yield)
(Note 23).
2. Notes
1.
The
ribofuranose and magnesium perchlorate hexahydrate were obtained from Aldrich Chemical Company, Inc.
, and used as received.
2.
2,6-Lutidine (99%) was obtained from Aldrich Chemical Company, Inc.
, in a
Sure/Seal bottle and used as received.
3.
Dichloromethane was freshly distilled from
calcium hydride.
4.
3-(Trifluoromethyl)benzoyl chloride was obtained from Acros Chemicals or Aldrich Chemical Company, Inc.
, and used as received.
5.
Silica gel (32-63 μm) was obtained from Fisher Scientific Company
.
6.
The physical properties are as follows:
[α]
D
21 +89.5° (CHCl
3,
c 0.1); IR (KBr) cm
−1: 1730
;
1H NMR (CDCl
3) δ: 4.71 (ABX, 2 H, J
AB = 12.1, J
AX = 3.5, J
BX = 3.1, Δν
AB = 32.6), 4.93 (m, 1 H), 5.79 (dd, 1 H, J = 6.5, 4.4), 5.88 (dd, 1 H, J = 6.5, 2.1), 6.91 (d, 1 H, J = 4.3), 7.31-7.61 (m, 10 H), 7.72 (d, 1 H, J = 4.3), 8.0-8.11 (m, 8 H)
;
13C NMR (CDCl
3) δ: 64.0 (t), 70.7 (d), 71.5 (d), 82.8 (d), 94.9 (d), 126.3, 126.4, 128.4, 128.5, 128.6, 128.9, 129.2, 129.3, 129.5, 129.7, 129.8, 130.0, 133.0, 133.4, 133.6, 133.7, 163.5, 165.1, 165.6, 166.0 (s)
, several aromatic signals were not resolved; exact mass calcd. for C
34H
25F
2O
9 (M
+-F) m/z 615.1480. found m/z 615.1473. Anal. Calcd for C
34H
25F
3O
9: C, 64.34; H, 3.97. Found: C, 63.90; H, 3.97.
7.
Chlorotrimethylsilane (99+%) was obtained from Aldrich Chemical Company, Inc.
, in a Sure/Seal bottle and used as received or distilled from
calcium hydride just prior to use.
8.
Benzene was freshly distilled from
sodium.
9.
Triethylamine (99+%) was obtained from Aldrich Chemical Company, Inc.
, and was freshly distilled from
calcium hydride.
10.
A plastic drying tube filled with Drierite was placed between the filter and the aspirator.
11.
The product was stored under
argon in a
desiccator and appears to be stable for months. This material exhibited the following spectral characteristics:
1H NMR (C
6D
6) δ: 0.3 (s, 9 H), 0.4 (s, 9 H), 1.8 (s, 3 H), 7.8 (s, 1 H)
; exact mass calcd. for C
11H
19N
2O
2 Si
2 (M
+- CH
3) m/z 255.0918. found m/z 255.0963.
12.
Anhydrous
acetonitrile was obtained from Aldrich Chemical Company, Inc.
in a Sure/Seal bottle and used as received.
13.
The white insoluble material is partially hydrolyzed
bis-TMS-thymine. The degree to which this suspension forms depends on the purity of the
bis-TMS-thymine and the water content of the flask and solvents.
14.
Neat
tin(IV) chloride (99%) was obtained from Aldrich Chemical Company, Inc.
in a Sure/Seal bottle and used as received.
15.
The physical properties are as follows:
[α]
D
21 −78° (CHCl
3,
c 0.1);
1H NMR (CDCl
3) δ: 1.54 (s, 3 H), 4.65 (m, 1 H), 4.70 (ABX, 2 H, J
AB = 12.3, J
AX = 2.6, J
BX = 3.5, Δν
AB = 69.5), 5.75 (t, 1 H, J = 6.1), 5.84 (dd, 1 H, J = 3.8, 6.0), 6.33 (d, 1 H, J = 6.1), 7.33-7.63 (m, 8 H), 7.93 (d, 2 H, J = 7.3), 7.99-8.17 (m, 4 H), 8.78 (br s, 1 H)
;
13C NMR (CDCl
3) δ: 12.4, 64.2, 71.6, 74.0, 80.8, 87.3, 112.5, 127.0, 128.7, 128.9, 129.1, 129.4, 129.6, 129.9, 130.0, 130.6, 133.4, 134.0, 135.1, 150.6, 163.7, 164.4, 165.7, 166.2
; IR (KBr) cm
−1: 3373, 3276, 3074, 1725, 1619, 1452, 1267, 1128, 1095, 713
. Anal. Calcd for C
32H
25F
3N
2O
9: C, 60.17; H, 3.95. Found: C, 60.26; H, 4.06.
16.
The submitters report an
82% yield for this reaction. The checkers recovered 0.5 g (9%) of starting material (mp 108-110°C) after recrystallization of material from early fractions of the column.
17.
The submitters purchased the reaction vessel from Ace Glass, Inc. (Catalog # 7841-15) and used a quartz immersion well purchased from Ace Glass, Inc. (Catalog # 7858-08) with a Pyrex filter and a Hanovia medium pressure, 450 W mercury lamp (Ace Glass, Inc. Catalog #7875). The checkers used the same apparatus except with a jacketed Pyrex immersion well in place of the quartz immersion well with a Pyrex filter.
18.
The solvent was previously degassed by bubbling UHP
argon through the solvent for 30 min using a gas sparger.
HPLC grade
2-propanol was obtained from Aldrich Chemical Company, Inc.
, and used as received. The submitters used deionized water and the checkers used HPLC grade water from Aldrich Chemical Company, Inc.
19.
The reaction assembly was immersed in a 5-gallon bucket of ice water. The reaction temperature was monitored and never went higher than 20°C. The checkers cooled the immersion well with recirculating water chilled to 10°C and used a 9-L bucket of ice water, and the internal temperature never went higher than 15°C.
20.
The rate of reaction is highly variable depending on the UV source. The reaction is easily monitored by TLC on silica gel using
ethyl acetate-
hexane (1:1) as the eluent and
p-anisaldehyde stain. The R
f values and staining colors of starting materials and products follow:
9-ethyl-3,6-dimethylcarbazole: R
f = 0.95 (gray);
3',5'-di-O-benzoyl-2'-O-[(3-trifluoromethyl)benzoyl]-5-methyluridine: R
f = 0.75 (pink-purple);
5'-O-benzoyl-3'-deoxythymidine: R
f = 0.55 (aqua blue);
3',5'-di-O-benzoylthymidine: R
f = 0.30 (brown-black).
21.
The submitters indicate that synthetic
3',5'-di-O-benzoylthymidine was identical in all respects to an authentic sample prepared by the benzoylation of
thymidine: IR (KBr) cm
−1: 1695
;
1H NMR (CDCl
3) δ: 1.62 (s, 3 H), 2.35-2.37 (m, 1 H), 2.67-2.73 (m, 1 H), 4.54 (d, 1 H, J = 2.0), 4.75 (ABX, 2 H, J
AB = 12.3, J
AX = 3.0, J
BX = 2.9, Δν
AB = 36.7), 5.66 (d, 1 H, J = 4.5), 6.47 (d, 1 H, J = 8.7, 5.6), 7.46-7.51 (m, 5 H), 7.60-7.65 (m, 2 H), 8.03-8.09 (m, 4 H), 8.50 (br s, 1 H)
;
13C NMR (CDCl
3) δ: 12.7 (q), 38.0 (t), 64.3 (t), 75.0 (d), 82.7 (d), 84.9 (d), 111.7 (s), 128.6 (d), 128.8 (d), 129.0 (s), 129.3 (s), 129.5 (d), 129.8 (d), 133.7 (d, 2C), 134.4 (d), 150.1 (s), 163.2 (s), 166.0 (s), 166.1 (s)
. Anal. Calcd for C
24H
22N
2O
7: C, 63.98; H, 4.93. Found: C, 64.07; H, 4.96.
22.
Synthetic
5'-O-benzoyl-3'-deoxythymidine was identical in all respects to an authentic sample prepared by the benzoylation of
3'-deoxythymidine: IR (KBr) cm
−1: 1694
;
1H NMR (CDCl
3) δ: 1.70 (d, 3 H, J = 0.9), 1.76-2.21 (m, 3 H), 2.44-2.51 (m, 1 H), 4.40-4.50 (m, 1 H), 4.53 (dd, 1 H, J = 12.2, 4.6), 4.65 (dd, 1 H, J = 12.2, 2.8), 6.1 (dd, 1 H, J = 6.4, 4.2), 7.35 (s, 1 H), 7.45 (t, 2 H, J = 7.5), 7.60 (t, 1 H, J = 7.4), 8.03 (d, 2 H, J = 7.1), 8.98 (br s, 1 H)
;
13C NMR (CDCl
3) δ: 12.4, 26.0, 32.3, 65.3, 78.4, 86.1, 110.7, 128.7, 129.6, 133.4, 133.5, 135.1, 150.3, 163.8, 166.0
.
23.
In one experiment, the checkers obtained 30%, 53%, and 1% of starting material (SM), monodeoxygenation product (MD) and dideoxygenation product (DD), respectively, after 7 hr of irradiation. In a second experiment, they obtained 3%, 57%, and 5% of SM, MD, and DD, respectively, after 10 hr of irradiation. The checkers also recovered
110 mg (
55%) of
9-ethyl-3,6-dimethylcarbazole (mp
56-58°C) from early fractions of the column.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Nucleosides and nucleoside analogs are a clinically proven class of medicinal agents possessing antiviral and anticancer activity.
6
7
8
9 The currently used sequence for the de novo synthesis of nucleosides involves the reaction of ribose tetraester with the appropriate silylated base under Lewis acid conditions (Vorbrüggen glycosylation).
6,7,8,9,10
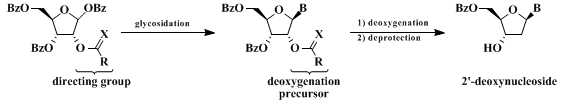
11 Presumably, reaction of ribose tetraester with Lewis acids give an oxacarbenium ion intermediate. Neighboring group participation of the α-C
2-ester group directs glycosylation exclusively to the desired β-face. In the absence of a directing C
2-group, mixtures of anomers are usually obtained. Robins developed an efficient procedure for the conversion of ribonucleosides to 2'-deoxyribonucleosides that relies on the simultaneous protection of the 3'- and 5'-hydroxyl groups with a bifunctional silylating reagent. The 2'-position is then deoxygenated via
tin hydride reduction of the corresponding
2'-phenoxythionocarbonate.
12
13
The strategy here was to develop a ribose glycosylation precursor in which the α-C
2-ester could serve as both a directing group for Vorbrüggen glycosylation and a deoxygenation precursor. This strategy was previously employed by Benner for the synthesis of potential antisense nucleosides with modified backbones.
14 In this work, a m-(trifluoromethyl)benzoyl group was used as the directing group/deoxygenation precursor. The submitters subsequently developed this strategy into a general method for the synthesis of β-2'-deoxyribonucleosides.
15 The selective deoxygenation of the 2'-m-(trifluoromethyl)benzoyl group is achieved via a photoinduced electron-transfer (PET) mechanism using stoichiometric
9-methylcarbazole (MCZ) as the electron donor.
16
The submitters recently reported the development of
9-ethyl-3,6-dimethylcarbazole (DMECZ) (see also accompanying procedure) as a donor that can be used in 10-20 mol %.
17 In their original report,
15 PET deoxygenation required 6-8 hr with stoichiometric MCZ at a substrate concentration of 1.4 mM. At higher substrate concentrations, the efficiency of PET deoxygenation dramatically decreased with MCZ. With 10-20 mol % of DMECZ as the donor, the deoxygenation of
3',5'-di-O-benzoyl-2'-O-[(3-trifluoromethyl)benzoyl]-5-methyluridine required 1-10 hr at a much higher substrate concentration, making the current procedure efficient and practical. DMECZ also shows improved reactivity and is able to deoxygenate benzoyl groups as well as m-(trifluoromethyl)benzoyl groups. This improved reactivity allowed the submitters to apply this strategy to the synthesis of α-2'-deoxyribonucleosides,
18 β-3'-deoxyribonucleosides and β-2',3'-dideoxyribonucleosides (Table).
TABLE
SYNTHESIS OF DEOXYRIBONUCLEOSIDES VIA PET DEOXYGENATION
|
|
Substrate (Ar= m-CF3C6H4−
|
Donor
|
Product
|
Yield
|
|
|
|
MCZ
|
|
70%
|
|
|
MCZ
|
|
53%
|
|
|
MCZ
|
|
44%
|
|
|
DMECZ
|
|
70%
|
|
|
DMECZ
|
|
58%
|
|
|
DMCZ
|
|
73%
|
|
|
DMECZ
|
|
69%
|
|
|
DMECZ
|
|
80%
|
|
|
DMECZ
|
|
81%
|
|
|
DMECZ
|
|
76%
|
|
|
DMECZ
|
|
60%
|
|
|
DMECZ
|
|
71%
|
|
|
DMECZ
|
|
65%
|
|
|
DMECZ
|
|
68%
|
|
Acknowledgment: This work was supported by Grant #DHP-172 from the American Cancer Society.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
3',5'-Di-O-benzoylthymidine:
Thymidine, 3',5'-dibenzoate (9): (35898-30-7)
1,3,5-O-Tribenzoyl-2-O-[(3-trifluoromethyl)benzoyl]-α-D-ribofuranose:
α-D-Ribofuranose, 1,3,5-tribenzoate 2-[3-(trifluoromethyl)benzoate (13); (145828-13-3)
1,3,5-Tri-O-benzoyl-α-D-ribofuranose: Aldrich:
α-D-Ribofuranose 1,3,5-tribenzoate:
Ribofuranose, 1,3,5-tribenzoate, α-D- (8);
α-D-Ribofuranose, 1,3,5-tribenzoate (9); (22224-41-5)
2,6-Lutidine (8);
Pyridine, 2,6-dimethyl- (9); (108-48-5)
3-(Trifluoromethyl)benzoyl chloride:
m-Toluoyl chloride, α,α,α-trifluoro- (8);
Benzoyl chloride, 3-(trifluoromethyl)- (9); (2251-65-2)
2,4-Bis(trimethylsilyloxy)-5-methylpyrimidine:
Pyrimidine, 5-methyl-2,4-bis(trimethylsiloxy)- (8);
Pyrimidine, 5-methyl-2,4-bis[(trimethylsilyl)oxy]- (9); (7288-28-0)
Thymine (8);
2,4(1H,3H)-Pyrimidinedione, 5-methyl- (9); (65-71-4)
Chlorotrimethylsilane:
Silane, chlorotrimethyl- (8,9); (75-77-4)
Benzene: CANCER SUSPECT AGENT (8,9); (71-43-2)
Trimethylamine (8);
Ethanamine, N,N-diethyl- (9); (121-44-8)
3',5'-Di-O-benzoyl-2'-O[(3-trifluoromethyl)benzoyl]-5-methyluridine:
Uridine, 5-methyl-, 3',5'-dibenzoate 2'-[3-(trifluoromethyl)benzoate] (13); (182004-59-7)
Acetonitrile: TOXIC (8,9); (75-05-8)
Tin(IV) chloride:
Tin chloride (8);
Stannane, tetrachloro- (9); (7646-78-8)
Magnesium perchlorate hexahydrate:
Perchloric acid, magnesium salt, hexahydrate (8,9); (13446-19-0)
9-Ethyl-3,6-dimethylcarbazole:
9H-Carbazole, 9-ethyl-3,6-dimethyl- (9); (51545-42-7)
5'-O-Benzoyl-3'-deoxythymidine:
Thymidine, 3'-deoxy-, 5'-benzoate (12); (122621-07-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved