Org. Synth. 2005, 82, 55
DOI: 10.15227/orgsyn.082.0055
IRIDIUM-CATALYZED SYNTHESIS OF VINYL ETHERS FROM ALCOHOLS AND VINYL ACETATE
[1-Methoxy-4-vinyloxybenzene]
Submitted by Tomotaka Hirabayashi, Satoshi Sakaguchi, and Yasutaka Ishii
1,2.
Checked by Paul W. Davies and Alois Fürstner.
1. Procedure
A 100-mL, two-necked round-bottomed flask is fitted with a magnetic stirbar, a reflux condenser connected to an argon/vacuum line, and a rubber septum. The equipment is flame dried under vacuum and then flushed with argon. Di-μ-chloro-bis(1,5-cyclooctadiene)diiridium(I), [Ir(cod)Cl]2, (0.34 g, 0.01 eq., 0.5 mmol) (Note 1) and sodium carbonate (3.18 g, 30 mmol, 0.6 eq.) (Note 2) are rapidly weighed in air and added to the flask which is resealed, evacuated and backfilled with argon. Toluene (50 mL) (Note 3), p-methoxyphenol (6.21 g, 50 mmol) (Note 4) and vinyl acetate (8.61 g, 100 mmol) (Note 5) are successively introduced and the flask is placed into a preheated oil-bath at 100°C with magnetic stirring. The reaction mixture changes from a yellow color after the addition of the vinyl acetate to a wine red color within 30 minutes of heating (Note 6) and continues to darken over the course of the reaction. After heating for 2 h, the reaction mixture is allowed to cool to ambient temperature before being transferred to a separatory funnel. Portions of EtOAc used to rinse the flask (3 × 50 mL) are added to the separatory funnel. The combined organic fractions are washed with water (3 × 100 mL) and brine (50 mL) before being dried over MgSO4. The solution is filtered, the cake is washed with EtOAc (2 × 20 mL), and the combined filtrates are evaporated (Note 7). The resulting crude product is applied to a pre-packed silica gel column (50 × 3.5 cm) (Note 8) and eluted with hexanes/EtOAc (4:1) giving 1-methoxy-4-vinyloxybenzene as a pale yellow liquid after evaporation of the solvent (6.85 g, 91%) (Notes 7, 9, 10).
2. Notes
1.
[Ir(cod)Cl]2 can be prepared according to the literature method.
3 This complex is commercially available from Aldrich Chemical Company, Inc. The checkers used
[Ir(cod)Cl]2 purchased from Strem without further purification.
2.
Na2CO3 purchased from Wako Pure Chemical Industries Ltd. was used as received. The checkers, however, found that the use of moist
Na2CO3 led to lower yields of the desired product and increased amounts of the acetate side-product. Well reproducible results were obtained by using
Na2CO3 dried under high vacuum (10
−4 Torr) at 80°C overnight before use.
3.
Toluene was dried by distillation over Na under Ar atmosphere.
4.
p-Methoxyphenol was purchased from Wako Pure Chemical Industried Ltd. or Acros and used as received.
5.
Vinyl acetate purchased from commercial suppliers (Wako Pure Chemical Industries Ltd., or Acros) was dried over MS 4 Å and distilled at normal pressure (bp 72°C) under Ar.
6.
This characteristic color change is much slower if insufficiently dried
Na2CO3 is used, thus leading to longer overall reaction times.
7.
To avoid any loss of product during the evaporation of the solvent, the vacuum was set to ≥ 20 mbar and the temperature of the water bath should not exceed 40°C.
8.
Silica gel (230-400 mesh) from Kanto Kagaku Reagent Division was used. The checkers used
Merck silica gel (230-400 mesh).
9.
A small amount (
<3%) of
1-acetoxy-4-methoxybenzene was observed as a side product. The checkers obtained somewhat larger amounts of this compound (
0.52 g,
6%).
10.
Analytical data:
1H NMR
pdf (400 MHz, CDCl
3) δ 6.97-6.84 (m, 4H), 6.59 (dd,
J = 6.2, 13.8 Hz, 1H), 4.64 (dd,
J = 1.7, 13.8 Hz, 1H), 4.34 (dd,
J = 1.7, 6.2 Hz, 1H), 3.79 (s, 3H);
13C NMR (101 MHz, CDCl
3) δ 155.8, 150.7, 149.6, 118.8, 114.8, 93.7, 55.8; IR (film) 2953, 2835, 1639, 1500, 1209, 1147, 1034, 954, 830 cm
−1. Anal. Calcd for C
9H
10O
2: C, 71.98; H 6.71; Found: C, 71.85; H, 6.67.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Vinyl ethers are conventionally prepared by mercury-catalyzed transfer vinylation,
4 base- or transition metal-catalyzed isomerization of allyl ethers,
5 or elimination reactions.
6 Quite recently, the synthesis of allyl and alkyl vinyl ethers by the transfer vinylation of alcohols with
butyl vinyl ether in the presence of a
palladium catalyst has been reported.
7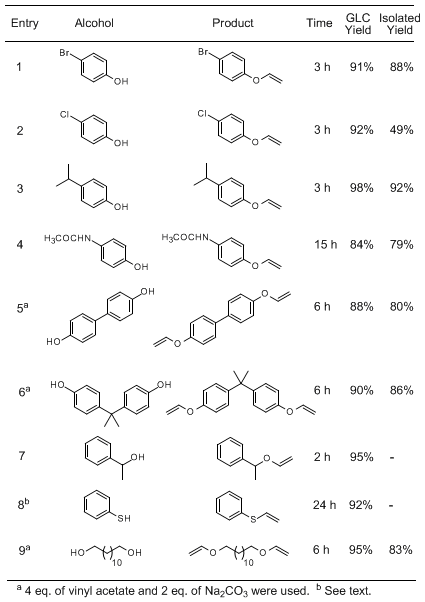
The present method uses commercially available
[Ir(cod)Cl]2 as the precatalyst for the synthesis of vinyl ethers which are very difficult to prepare otherwise.
2 The reaction is thought to proceed through an addition-elimination sequence of alcohol and
acetic acid in the presence of the iridium complex as a catalyst and
Na2CO3 as the base. Representative examples are compiled in the 1. The method can also be applied to the synthesis of vinyl ethers derived from secondary alcohols (entry 7) and holds promise for the preparation of alkyl vinyl ethers (entry 9). Although sulfur compounds frequently inhibit transition metal-catalyzed reactions,
thiophenol reacts with
vinyl acetate to form
phenylvinyl thioether in excellent yield (entry 8); this compound, however, is unstable in air and is therefore difficult to isolate in analytically pure form (~15 %).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Di-μ-chloro-bis(1,5-cyclooctadiene)diiridium(I):
Iridium, di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]di-; (12112-67-3)
Sodium carbonate:
Carbonic acid disodium salt; (497-19-8)
Toluene:
Benzene, methyl-; (108-88-3)
p-Methoxyphenol:
Phenol, 4-methoxy-; (150-76-5)
Vinyl acetate:
Acetic acid ethenyl ester; (108-05-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved