Org. Synth. 2012, 89, 307-310
DOI: 10.15227/orgsyn.089.0307
Discussion Addendum for:
[Iridium-catalyzed Synthesis of Vinyl Ethers from Alcohols and Vinyl Acetate]
Submitted by Yasushi Obora* and Yasutaka Ishii
*1.
Discussion
The synthesis of vinyl ethers by vinyl transfer from vinyl esters to alcohols constitutes the basis for much useful methodology.
The originally reported iridium-catalyzed process provides a versatile and practical route to access vinyl alcohols.
2 This methodology was successfully expanded to facilitate allyl transfer from allyl acetates to alkyl alcohols, providing allyl ethers as products.
3 In addition, as a successful application of this protocol, the one-pot synthesis of γ,δ-unsaturated carbonyl compounds from allyl alcohols and vinyl acetates was achieved through in situ iridium-catalyzed formation of allylic vinyl ethers followed by Claisen rearrangement.
4 The above-mentioned advances will be summarized here.
Scope of Allylation of Alcohols from Allyl Acetates
Vinyl ethers and allyl ethers are important classes of compounds that have been used in polymer synthesis and in the pharmaceutical chemistry,
5 as well as starting materials for Claisen rearrangements,
6 cycloadditions,
7 hydroformylations,
8 and Mizoroki-Heck reactions,
9 etc.
We found that the iridium cationic complex [Ir(cod)2]+BF4− catalyzed the allylation of alcohols with allyl acetate to afford allyl ethers as products.3 For instance, the reaction of allyl acetate with n-octyl alcohol in the presence of a catalytic amount of [Ir(cod)2]+BF4− complex afforded allyl octyl ether in quantitative yield (Figure 1).
Figure 1. Ir-catalyzed reaction of n-octyl alcohol with allyl acetate to allyl octyl ether
Application of the Vinyl Transfer Methodology in Organic Synthesis
We previously reported the rearrangement of allyl homoallyl ethers to γ,δ-unsaturated aldehydes induced by the [IrCl(cod)]
2 complex.
10 Therefore, this protocol would serve to a useful route for the formation of γ,δ-unsaturated carbonyl compounds from allyl alcohols with vinyl or isopropenyl acetate through the formation of vinyl ethers as the key intermediate.
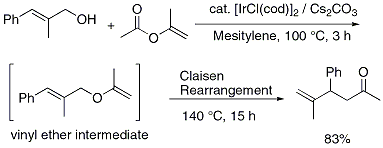
4 For example, the reaction of
trans-2-methyl-3-phenyl-2-propen-1-ol with isopropenyl acetate in the presence of [IrCl(cod)]
2 catalyst combined with Cs
2CO
3 at 100 °C for 3 h followed by heating at 140 °C for 15 h afforded 5-methyl-4-phenyl-5-hexen-2-one in 83% yield (Figure 2).
The reaction is thought to proceed through the Claisen rearrangement of the in situ generated allylic vinyl ether.
Figure 2. Ir-catalyzed one-pot synthesis of γ,δ-unsaturated carbonyl compounds
 |
Yasutaka Ishii was born in Osaka, Japan, in 1941.
He received his B.A.
(1964) and M.S.
(1967).
In 1967, he was appointed Assistant Professor at Kansai University.
In 1971, he received his Ph.D.
degree under the supervision of Prof.
Masaya Ogawa.
He was a postdoctoral fellow with Professor Louis S.
Hegedus at Colorado State University (1980-1981).
In 1990 he was appointed as Professor at Kansai University.
Since 2009, he has been an Emeritus Professor at Kansai University.
His research interests include the development of practical oxidation reactions using molecular oxygen and hydrogen peroxide, homogeneous catalysis directed towards organic synthesis. |
 |
Yasushi Obora was born in 1969 in Shizuoka and received his B.Sc.
(1991) and Ph.D.
degree (1995) from Gifu University.
After working as a postdoctoral fellow (1995-1997) at Northwestern University with Professor T.
J.
Marks, he moved to the National Institute of Materials and Chemical Research, AIST (1997-1999).
In 1999, he moved to Catalysis Research Center at Hokkaido University as Research Associate.
In 2006, he was appointed as an Associate Professor at Kansai University.
His research interests include the development of new homogeneous catalysis and organometallic chemistry. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved